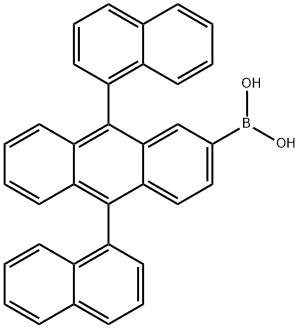
(9,10-di(naphthalene-1-yl)anthracen-2-yl)boronic acid synthesis
- Product Name:(9,10-di(naphthalene-1-yl)anthracen-2-yl)boronic acid
- CAS Number:867044-35-7
- Molecular formula:C34H23BO2
- Molecular Weight:474.36

5419-55-6
389 suppliers
$12.00/5g
929031-39-0
77 suppliers
$45.00/10mg

7732-18-5
504 suppliers
$12.69/100ml

867044-35-7
46 suppliers
$60.00/10 mg
Yield: 66.2%
Reaction Conditions:
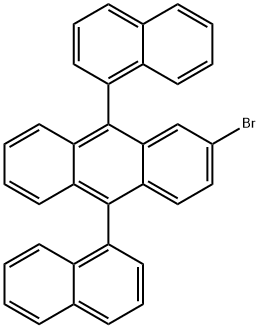
Stage #1:2-bromo-9,10-bis(naphthalen-1-yl)anthracene with n-butyllithium in tetrahydrofuran at -78; for 1.5 h;
Stage #2:Triisopropyl borate in tetrahydrofuran at 20; for 24 h;
Stage #3:water with hydrogenchloride in tetrahydrofuran
Steps:
9.9-2 Synthesis Example 9- (2): Synthesis of 9,10-di (1'-naphthyl) -2-anthracenylboronic acid
While stirring a 500 ml round bottom flask, 25.00 g (0.049 mol) and 250 ml (THF) of compound (M-1) prepared in Synthesis 9-1 were added.After the temperature was dropped to -78 ° C, 33.7 ml (0.054 mol) of n-BuLi was slowly added dropwise.After the dropwise addition was stirred for 1 hour and 30 minutes, 16.93 g (0.074 mol) of triisopropyl borate was added dropwise.After standing for 10 minutes, the mixture was stirred at room temperature for 1 day. When the reaction is over, add 100 ml of 2N hydrochloric acid to make it acidic, extract with ethyl acetate and water,After removing water with MgSO 4, it was concentrated. The concentrated solution was recrystallized from hexane.As a result, 15.4 g (0.03 mol, yield 66.2%) of white solid was obtained.
References:
SFC Ltd.;Che Jong-tae;Hwang Seok-gwang;Choi A-ram;Kim Seong-hun KR101553561, 2015, B1 Location in patent:Paragraph 0252; 0257-0260
572-83-8
218 suppliers
$6.00/1g

867044-35-7
46 suppliers
$60.00/10 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

867044-35-7
46 suppliers
$60.00/10 mg