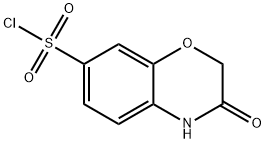
3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonyl chloride synthesis
- Product Name:3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonyl chloride
- CAS Number:868962-24-7
- Molecular formula:C8H6ClNO4S
- Molecular Weight:247.66
26215-14-5
75 suppliers
$34.00/500mg
![3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonyl chloride](/CAS/20180703/GIF/868962-24-7.gif)
868962-24-7
10 suppliers
inquiry
Yield:868962-24-7 52%
Reaction Conditions:
Stage #1: 7-amino-2H-1,4-benzoxazin-3(4H)-onewith hydrogenchloride;sodium nitrite in water;acetic acid;acetonitrile at 0; for 1 h;
Stage #2: with sulfur dioxide;copper dichloride in water;acetic acid;acetonitrile at 0;
Steps:
13 Synthesis of 3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonyl Chloride
Synthesis of 3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonyl Chloride Into a 2 L 3-necked round-bottom flask, was placed a solution of 7-amino-2H-benzo[b][1,4]oxazin-3(4H)-one (13.5 g, 78.20 mmol, 1.00 equiv, 95%) in CH3CN (1 L). To the above was added HOAc (100 g) dropwise with stirring, while cooling to a temperature of 0° C. To the above was added HCl (50 g, 36.5%) dropwise with stirring, while cooling to a temperature of 0° C. To the above was added NaNO2 (6.25 g, 90.58 mmol, 1.00 equiv) in several batches, while cooling to a temperature of 0° C. The resulting solution was allowed to react, with stirring, for 60 min while the temperature was maintained at 0° C. in a bath of H2O/ice. This was followed by and maintained with an atmosphere of SO2, the resulting solution was allowed to react, with stirring, for an additional 2 h while the temperature was maintained at 0° C. in a bath of H2O/ice. To the mixture was added CuCl2.2H2O (14 g, 82.12 mmol, 1.00 equiv), while cooling to a temperature of 0° C. The resulting solution was allowed to react, with stirring, maintained with an atmosphere of sulfur dioxide for an additional 2 h while the temperature was maintained at 0° C. in a bath of H2O/ice. The resulting solution was allowed to react, with stirring, overnight while the temperature was maintained at room temperature. The reaction progress was monitored by TLC (PE:EtOAc=1:1). The reaction mixture was then quenched by the adding 1 L of H2O/ice. The resulting solution was extracted 4 times with 2 L of dichloromethane and the organic layers combined. The resulting mixture was washed 5 times with 1 L of brine. The mixture was dried over MgSO4. A filtration was performed. The filtrate was concentrated by evaporation under vacuum using a rotary evaporator to a small volume. A filtration was performed. After filtrated and washed with dichloromethane, this resulted in 10.05 g (52%) of 3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonyl chloride as a yellow solid. LC-MS (m/z): [M+H]+ calcd for C8H7ClNO4S: 248, found: 248 1H NMR (300MHz, CDCl3, δ) 4.74 (2H, s), 6.98 (1H, d), 7.66 (1H, s), 7.70 (1H, d), 8.00 (1H, s).
References:
US2008/318941,2008,A1 Location in patent:Page/Page column 30