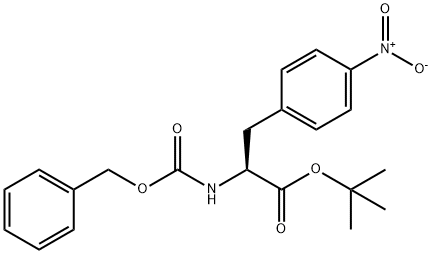
(S)-tert-Butyl 2-(((benzyloxy)carbonyl)-amino)-3-(4-nitrophenyl)propanoate synthesis
- Product Name:(S)-tert-Butyl 2-(((benzyloxy)carbonyl)-amino)-3-(4-nitrophenyl)propanoate
- CAS Number:869882-71-3
- Molecular formula:C21H24N2O6
- Molecular Weight:400.43
Yield:869882-71-3 83%
Reaction Conditions:
with sulfuric acid in 1,4-dioxane at -20 - 20; for 20 h;
Steps:
19
The Cbz-4-nitrophenylalanine, 19D, (75 g, 0.22 mol) was dissolved in dioxane (300 mL). The resulted stirred solution was cooled in dry ice bath to -20° C. (internal). The liquefied isobutylene (approx. 290 mL) was added followed by conc. sulfuric acid (35 mL) added in three equal portions, 30 min apart. The addition of acid is a very exothermic process, accompanied by substantial degree of polymerization. Efficient mechanical stirring is essential at this stage. Resulted mixture was stirred for 20 hr, allowing to warm up to ambient temperature then was cautiously poured into sat. aq. sodium carbonate solution (2 L) and diluted with ethyl acetate (600 mL). Organic layer was separated and aqueous layer was extracted with ethyl acetate (2×200 mL). Combined extracts were washed with water and dried with sodium sulfate. Obtained solution was filtered and evaporated to dryness. Residue was taken up in ethyl acetate/hexane mixture (500 mL; 1:1) and filtered through plug of silica gel (ca. 2×2 inches). Silica was rinsed with additional amount of the same solvent (2 L total) and filtrates were evaporated to give fully protected 4-nitrophenylalanine as a viscous oil, 73 g (83% after two steps) 19E. 1H-NMR, CDCl3, (δ): 8.12 (d, 2H, J=8.4 Hz), 7.36 (m, 7H), 5.35 (m, 1H), 5.10 (m, 2H), 4.57 (m, 1H), 3.31 (m, 2H), 1.43 (s, 9H). 13C-NMR, CDCl3, (δ): 169.7, 155.3, 146.9, 143.9, 136.0, 130.2, 128.4, 128.2, 128.0, 123.3, 82.9, 66.9, 54.7, 38.2, 31.4, 27.8, 13.9.
References:
US2005/261324,2005,A1 Location in patent:Page/Page column 36-37
![L-Phenylalanine, 4-nitro-N-[(phenylMethoxy)carbonyl]-](/CAS/GIF/17224-90-7.gif)