
(1R,3R,5S)-tert-butyl 3-hydroxy-3-methyl-8-azabicyclo[3.2.1]octane-8-carboxylate synthesis
- Product Name:(1R,3R,5S)-tert-butyl 3-hydroxy-3-methyl-8-azabicyclo[3.2.1]octane-8-carboxylate
- CAS Number:870889-20-6
- Molecular formula:C13H23NO3
- Molecular Weight:241.33
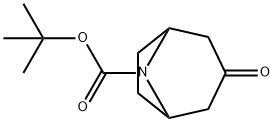
185099-67-6
246 suppliers
$5.00/250mg
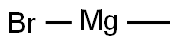
75-16-1
274 suppliers
$12.00/10ml
![(1R,3R,5S)-tert-butyl 3-hydroxy-3-methyl-8-azabicyclo[3.2.1]octane-8-carboxylate](/CAS/GIF/870889-20-6.gif)
870889-20-6
21 suppliers
inquiry
Yield:870889-20-6 31%
Reaction Conditions:
with lithium bromide in tetrahydrofuran at 50; for 2 h;
Steps:
A solution of methyl magnesium bromide in diethyl ether (3 M, 3.7 mL, 11.09 mmol) was diluted with anhydrous THF (5 mL). Lithium bromide (1.93 g, 22.1 mmol) was slowly added to the solution at rt, followed by addition of a solution of Boc- nortropinone (500 mg, 2.21 mmol) in anhydrous THF (5 mL). The reaction mixture was stirred at 50 °C for 2 hours and stirring was continued overnight at rt. The reaction was quenched with water and the mixture partitioned between ethyl acetate and water. The organic layer was dried over sodium sulfate, filtered and evaporated to dryness. Purification of the residue by silica gel column chromatography, eluting with a mixture of ethyl acetate and n-heptane (50: 50), afforded 3-hydroxy-3-methyl-8-azabicyclo[3.2.1]octane-8- carboxylic acid tert-butyl ester (168 mg, 31%).
References:
WO2005/115361,2005,A2 Location in patent:Page/Page column 85