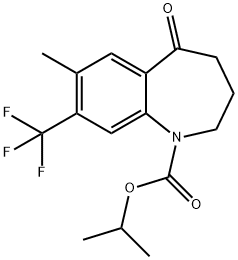
1H-1-Benzazepine-1-carboxylic acid, 2,3,4,5-tetrahydro-7-Methyl-5-oxo-8-(trifluoroMethyl)-, 1-Methylethyl ester synthesis
- Product Name:1H-1-Benzazepine-1-carboxylic acid, 2,3,4,5-tetrahydro-7-Methyl-5-oxo-8-(trifluoroMethyl)-, 1-Methylethyl ester
- CAS Number:872624-57-2
- Molecular formula:C16H18F3NO3
- Molecular Weight:329.31

872624-56-1
0 suppliers
inquiry

872624-57-2
7 suppliers
inquiry
Yield:872624-57-2 87%
Reaction Conditions:
with hydrogenchloride;water;acetic acid at 20 - 88; for 24 h;
Steps:
3.8
Step 8; Preparation of Isopropyl 7-methyl-5-oxo-8-trifluoromethyl-2,3,4,5-tetrahydrobenzo [b] azepine-1 -carboxylate; Add 5 M HC1 (2.115 L) to a solution of (+/-)-l-isopropyl-4-methyl-7-methyl-5-oxo-8-trifluoromethyl-2,3,4,5-tetrahydrobenzo [b]azepine- 1,4-dicarboxylate (197.2 g, 509mmol) in glacial acetic acid (500 mL) at room temperature under an atmosphere ofnitrogen and heat the stirred mixture at 87 - 88 °C for 24 h. Allow the mixture to cool toroom temperature with stirring and filter the suspension. Wash the collected solids withwater and pull dry. Dry under vacuum at 45 °C to give the title compound as a creamsolid (145.4 g, 87%).
References:
WO2006/2342,2006,A1 Location in patent:Page/Page column 59