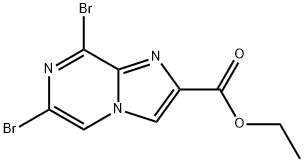
6,8-Dibromo-imidazo[1,2-a]pyrazine-2-carboxylic acid ethyl ester synthesis
- Product Name:6,8-Dibromo-imidazo[1,2-a]pyrazine-2-carboxylic acid ethyl ester
- CAS Number:87597-21-5
- Molecular formula:C9H7Br2N3O2
- Molecular Weight:348.98
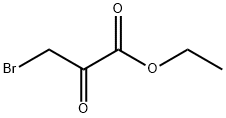
70-23-5
24241-18-7
![6,8-Dibromo-imidazo[1,2-a]pyrazine-2-carboxylic acid ethyl ester](/CAS2/GIF/87597-21-5.gif)
87597-21-5
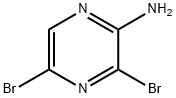
Step A: Preparation of ethyl 6,8-dibromoimidazo[1,2-a]pyrazine-2-carboxylate At room temperature, 2-amino-3,5-dibromopyrazine (20 g, 79 mmol) was dissolved in dimethyl carbonate (133 mL) and stirred until completely dissolved. Subsequently, ethyl 3-bromo-2-oxopropionate (17.14 g, 79 mmol) was added to the reaction system in one go. The reaction mixture was heated to 110 °C with continuous stirring for 3 hours. Upon completion of the reaction, the mixture was cooled to room temperature and stirring was continued overnight. Water and dichloromethane (DCM) were added to the reaction mixture, the aqueous phase was separated and extracted with DCM. The organic phases were combined, washed with water, dried over anhydrous sodium sulfate (Na2SO4), filtered and the solvent evaporated. Purification by fast column chromatography afforded 13.95 g (50.6% yield) of ethyl 6,8-dibromoimidazo[1,2-a]pyrazine-2-carboxylate, the target compound. The product was characterized by 1H-NMR (300 MHz, CDCl3): δ= 8.30 (s, 1H), 8.27 (s, 1H), 4.48 (q, 2H), 1.43 (t, 3H) ppm.

70-23-5
318 suppliers
$6.00/5g

24241-18-7
447 suppliers
$6.00/5g
![6,8-Dibromo-imidazo[1,2-a]pyrazine-2-carboxylic acid ethyl ester](/CAS2/GIF/87597-21-5.gif)
87597-21-5
48 suppliers
$101.00/250mg
Yield:87597-21-5 79%
Reaction Conditions:
in 1,2-dimethoxyethane at 20 - 80; for 24 h;
Steps:
Ethyl 6,8-dibromoimidazo[l,2-a]pyrazine-2-carboxylate (Sl.l)
To a mixture of 2-amino-3,5-dibromopyrazine (3.0 g, 11.86 mmol) in anhydrous DME (12 mL) was added ethyl bromopyruvate (2 mL ~ 90% pure, 14.34 mmol) at r.t. The reaction mixture was heated at 80 °C for 24 hr. The mixture was then diluted with DCM, washed with sat. NaHC03 solution (x3), dried over MgSO4 and the solvent concentrated in vacuo. The resi- due was then triturated with cold diethyl ether and the solid was collected via filtration to give the title compound as a fawn color powder (3.26 g, 79%). This material was used in the next step without further purification. 1H NMR (400MHz, CDC13) d 8.29 (s, 1H), 8.25 (s, 1H), 4.49 (q, J = 7.1 Hz, 2H), 1.44 (t, J = 7.1 Hz, 3H). LCMS: (0 - 100 % Acetonitrile over 5 min, 0.1% formic acid throughout) Rf = 3.20 min, (ESI) m/z: 349.7 (M+H, 100%).
References:
WO2021/243421,2021,A1 Location in patent:Page/Page column 89; 110; 111
65868-37-3
8 suppliers
inquiry

24241-18-7
447 suppliers
$6.00/5g
![6,8-Dibromo-imidazo[1,2-a]pyrazine-2-carboxylic acid ethyl ester](/CAS2/GIF/87597-21-5.gif)
87597-21-5
48 suppliers
$101.00/250mg