
(R)-tert-Butyl 1-(3-methoxybenzyl)pyrrolidin-3-ylcarbamate synthesis
- Product Name:(R)-tert-Butyl 1-(3-methoxybenzyl)pyrrolidin-3-ylcarbamate
- CAS Number:876161-75-0
- Molecular formula:C17H26N2O3
- Molecular Weight:306.4
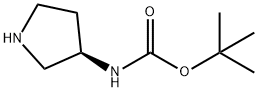
122536-77-0
321 suppliers
$9.00/1g
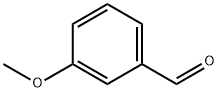
591-31-1
432 suppliers
$10.00/5g

876161-75-0
7 suppliers
$309.00/1g
Yield:-
Reaction Conditions:
Stage #1: 3-N-tert-butoxycarbonyl-3-(R)-aminopyrrolidine;3-methoxy-benzaldehydewith sodium tris(acetoxy)borohydride in dichloromethane at 20; for 2 h;
Stage #2: with water;sodium hydrogencarbonate in dichloromethane; for 0.333333 h;
Steps:
450
Preparation 450; To a mixture of tert-butyl (3R)-3-pyrrolidinylcarbamate (450mg) and3-methoxybenzaldehyde (352uL) in CH2Cl2 (4.5mL) was added sodiumtriacetoxyborohydride (922mg), which was stirred at roomtemperature for 2 hours. To the resultant was added sat. NaHCO3 aq.,Which was stirred for 20 min. The mixture was extracted with CH2C12.The organic phase was washed with brine and dried over Na2SO4,filtered, and evaporated in vacuo. The residue was purified bycolumn chromatography on silica gel to give tert-butyl [(3R)-l-(3-methoxybenzyl)-3-pyrrolidinyl]carbamate (744mg) as a off-whitesolid.XH NMR (200 Mz, CDC13) d 1.43 (9H, s), 1.49-1.72 (1H, m), 2.16-2.46(2H, m), 2.58-2.72 (2H, m), 2.80-2.98 (1H, m), 3.63 (2H, br. s),3.82 (3H, s), 4.08-4.31 (1H, br), 4.90-5.14 (1H, br), 6.76-6.95 (3H,m), 7.24 (1H, t, J ? 8.1 Hz);MS (ES+) m/z 307 (M+l).
References:
WO2006/16680,2006,A1 Location in patent:Page/Page column 184