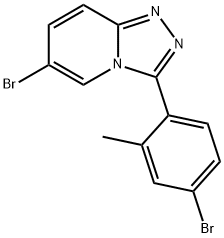
6-Bromo-3-(4-bromo-2-methylphenyl)-[1,2,4]triazolo[4,3-a]pyridine synthesis
- Product Name:6-Bromo-3-(4-bromo-2-methylphenyl)-[1,2,4]triazolo[4,3-a]pyridine
- CAS Number:876373-12-5
- Molecular formula:C13H9Br2N3
- Molecular Weight:367.04
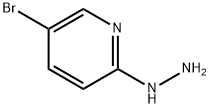
77992-44-0
219 suppliers
$8.00/1g
![6-Bromo-3-(4-bromo-2-methylphenyl)-[1,2,4]triazolo[4,3-a]pyridine](/CAS/20180703/GIF/876373-12-5.gif)
876373-12-5
3 suppliers
inquiry
Yield:876373-12-5 18%
Reaction Conditions:
Stage #1: 4-bromo-2-methylbenzoic acidwith oxalyl dichloride;N-ethyl-N,N-diisopropylamine in 1,4-dioxane at 20; for 2 h;
Stage #2: 5-bromo-2-hydrazinylpyridinewith N-ethyl-N,N-diisopropylamine in 1,4-dioxane;toluene at 20; for 0.25 h;
Stage #3: with trichlorophosphate in 1,4-dioxane;toluene at 95;
Steps:
45.1
4-Bromo-2-methylbenzoic acid (30.5 g, 142 mmol) was dissolved in 225 ml of 1 ,4- dioxane and diisopropylethylamine (26.9 ml, 170 mmol), then oxalyl chloride (13.6 ml, 156 mmol) was added dropwise and stirred at room temperature for 2 hours. The solution was then added dropwise to a suspension of 5-bromo-2-hydrazinopyridine (26.7 g, 142 mmol) in diisopropylethylamine (29.6 ml, 170 mmol) and 300 ml of 1,4-dioxane and 150 ml of toluene at room temperature. After 15 minutes, phosphorus oxychloride (28.6 ml, 312 mmol) was added and the reaction stirred at 95 0C overnight. The reaction was cooled, evaporated to about half the solvent volume and quenched with 500 ml of a NaHCO3 solution. The reaction mixture was extracted 2 times with 500 ml of ethyl acetate and the combined organic layers were washed with 500 ml of a NH4CI solution and 500 ml of brine, dried over MgSO4 and evaporated. The resulting residue was purified using silica gel chromatography to obtain a solid (9.12 g, 18% yield). 1H NMR (400 MHz, DMF-Cf7) δ 8.57 (s, 1H), 7.87 (d, J = 9.7 Hz, 1H), 7.74 (s, 1H), 7.66 - 7.61 (m, 2H), 7.57 (dd, J = 9.7, 1.7 Hz, 1H), 2.28 (s, 3H); LC/MS, tr = 2.43 minutes (5 to 95% acetonitrile/water over 5 minutes at 1 ml/min, at 254 nm, at 500C), ES-MS m/z 366 (M+H).
References:
WO2006/18735,2006,A2 Location in patent:Page/Page column 88