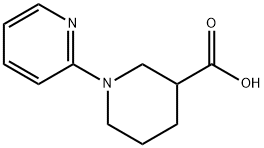
3,4,5,6-TETRAHYDRO-2H-[1,2']BIPYRIDINYL-3-CARBOXYLIC ACID synthesis
- Product Name:3,4,5,6-TETRAHYDRO-2H-[1,2']BIPYRIDINYL-3-CARBOXYLIC ACID
- CAS Number:876718-04-6
- Molecular formula:C11H14N2O2
- Molecular Weight:206.24
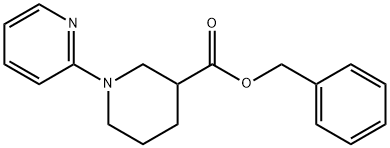
939410-76-1
1 suppliers
inquiry
![3,4,5,6-TETRAHYDRO-2H-[1,2']BIPYRIDINYL-3-CARBOXYLIC ACID](/CAS/GIF/876718-04-6.gif)
876718-04-6
41 suppliers
$65.00/50mg
Yield:-
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethyl acetate; under 760.051 Torr; for 48 h;
Steps:
31
Example 32: N-((2S,3R)-3-hydroxy-4-((S)-6-isopropyI-2,2-spirocyclopentyIchroman-4-ylamino)-l- phenylbutan-2-yl)-l-(pyridin-2-yl)piperidine-3-carboxamide. Pure benzyl piperidine-3-carboxylate (170 mg, 0.776 mmol; Example 31) was placed in a microwave tube. DIPEA (0.50 mL), tert-BuOH (1.5 mL) and 2- fluoropyridine (Aldrich, 0.40 mL) were added to the tube via syringes before the tube was subjected to microwave irradiation at 180 °Cfor 20 min. LC/MS showed partial conversion to the desired pyridine derivative. The tube was subjected to further microwave irradiation at 2200C for 20min and then it was allowed to cool to room temperature. The volatiles were removed by rotary evaporation to provide 229 mg of crude oil which was dissolved in MeOH (8.0 mL) and then subjected to reverse phase HPLC. The pure fractions were poured into Na2CC>3 (10%, 75 mL) and extracted with EtOAc (3 x 50 mL), washed with brine (sat, 150 mL), dried with sodium sulfate, filtered and concentrated to provide 90 mg of benzyl l-(pyridin-2-yl)piperidine-3-carboxylate. MS m/z: 297 (M+l)The benzyl ester from above was dissolved in EtOAc (30 mL) and 10% Pd/C (20 mg) was added in one portion. A balloon of H2 was bubbled through the reaction before it was allowed to stir under 1 atm OfH2 for 48 h. The reaction was filtered through a plug of celite and concentrated to provide 58 mg of clean 1 -(pyridin-2-yl)piperidine-3- carboxylic acid. MS m/z: 207 (M+l)The l-(pyridin-2-yl)piperidine-3-carboxylic acid was dissolved in DMF (1.0 mL) before DIPEA (0.1 mL), (2R,3 S)-3-amino- 1 -((S)-6-isopropyl-2,2- spirocyclopentylchroman-4-ylamino)-4-phenylbutan-2-ol, bis hydrochloride salt (58 mg), and HATU (50 mg) were added to the reaction. The reaction was allowed to stir for 1 h and then loaded directly to reverse phase HPLC. The pure fractions were poured into Na2CO3 (10%, 100 mL) and extracted with EtOAc (3 x 75 mL). The combined organics were washed with brine (sat 100 mL) and dried with sodium sulfate. The solution was filtered and concentrated to provide the title compound. MS m/z: 597.4 (M+l)
References:
WO2007/62007,2007,A1 Location in patent:Page/Page column 89-90