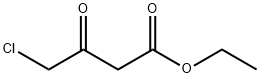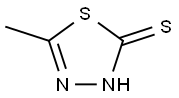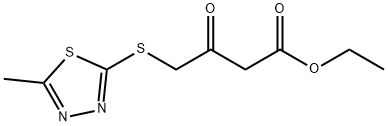
Ethyl 4-[(5-methyl-1,3,4-thiadiazol-2-yl)thio]-3-oxobutanoate synthesis
- Product Name:Ethyl 4-[(5-methyl-1,3,4-thiadiazol-2-yl)thio]-3-oxobutanoate
- CAS Number:877052-29-4
- Molecular formula:C9H12N2O3S2
- Molecular Weight:260.33
Yield:877052-29-4 81%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 60; for 2 h;Inert atmosphere;
Steps:
6.a Step a
To a solution of 5-methyl-l,3,4-thiadiazole-2-thiol (10.0 g, 75.6 mmol) and ethyl 4-chloro-3-oxobutanoate (14.9 g, 90.5 mmol) in DMF (100 mL) was added K2CO3 (15.7 g, 113.6 mmol) at room temperature. The reaction mixture was heated at 60 °C under nitrogen atmosphere for 2 h at which time it was cooled and diluted with CH2CI2 (500 mL) and washed with brine (250 mL x 3). The separated organic phase was dried over anhydrous Na2S04, filtered and concentrated under vacuum. The residue was purified by silica gel column chromatography, eluting with p-ether and ethyl acetate (10 : 1 to 1 : 1) to afford ethyl 4- [(5 -methyl- 1,3,4- thiadiazol-2-yl)sulfanyl]-3-oxobutanoate (16 g, 61 mmol, yield 81%) as an off-white solid: LCMS (ESI) calc'd C9H12N2O3S2 for [M+H]+: 261, found 261; NMR (300 MHz, DMSO-^e) δ ppm 4.47 (s, 2H), 4.10 (q, J= 7.1 Hz, 2H), 3.79 (s, 2H), 2.67 (s, 3H), 1.19 (t, J= 7.1 Hz, 3H).
References:
WO2019/55540,2019,A1 Location in patent:Paragraph 0203