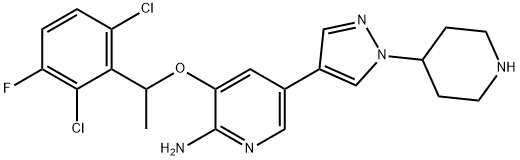
3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine synthesis
- Product Name:3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine
- CAS Number:877400-66-3
- Molecular formula:C21H22Cl2FN5O
- Molecular Weight:450.34
![1-Piperidinecarboxylic acid, 4-[4-[6-amino-5-[1-(2,6-dichloro-3-fluorophenyl)ethoxy]-3-pyridinyl]-1H-pyrazol-1-yl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/877401-42-8.gif)
877401-42-8
0 suppliers
inquiry

877400-66-3
29 suppliers
$60.00/1mg
Yield:877400-66-3 89%
Reaction Conditions:
Stage #1: tert-butyl 4-(4-(6-amino-5-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-3-yl)-1H-pyrazol-1-yl)piperidine-1-carboxylatewith hydrogenchloride in 1,4-dioxane;dichloromethane at 20;
Stage #2: with sodium hydrogencarbonate in 1,4-dioxane;dichloromethane;water; pH=8;
Steps:
1.6 Step 6. 3-(l-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(l-(piperidin-4-yl)-lH- pyrazol-4-yl)pyridin-2-amine (Compound 6).
To a solution of tert-butyl 4-(4-(6-amino-5-(l-(2,6-dichloro-3-fluorophenyl) ethoxy)pyridin-3-yl)-lH-pyrazol-l-yl)piperidine-l-carboxylate (330 mg, 0.6 mmol, 1.0 eq) in DCM (20 mL) was added saturated dioxane of HC1 (7 mL). The reaction mixture was stirred at rt overnight until TLC indicated the consumption of starting material. The pH of the reaction mixture was adjusted to 8 by saturated bicarbonate sodium. The aqueous was extracted with ethyl acetate (8x20 mL), the combined organic layers were washed with brine, dried (MgS04), filtered, and concentrated. The crude product was purified by column chromatography on silica gel to give the title compound 240 mg (89% yield).
References:
WO2013/41038,2013,A1 Location in patent:Page/Page column 24
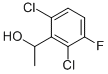
756520-66-8
91 suppliers
$10.00/1g

877400-66-3
29 suppliers
$60.00/1mg
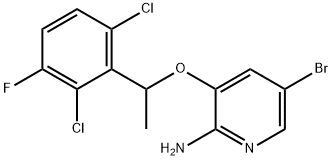
756503-69-2
19 suppliers
inquiry

877400-66-3
29 suppliers
$60.00/1mg
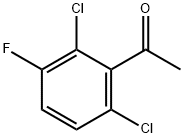
290835-85-7
263 suppliers
$6.00/10g

877400-66-3
29 suppliers
$60.00/1mg
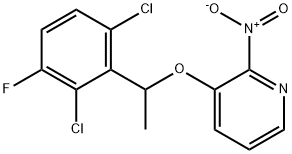
756521-08-1
15 suppliers
inquiry

877400-66-3
29 suppliers
$60.00/1mg