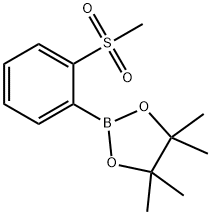
4,4,5,5-Tetramethyl-2-(2-(methylsulfonyl)phenyl)-1,3,2-dioxaborolane synthesis
- Product Name:4,4,5,5-Tetramethyl-2-(2-(methylsulfonyl)phenyl)-1,3,2-dioxaborolane
- CAS Number:879648-22-3
- Molecular formula:C13H19BO4S
- Molecular Weight:282.16
33951-33-6
79 suppliers
$21.00/1g

73183-34-3
557 suppliers
$6.00/5g

879648-22-3
48 suppliers
inquiry
Yield:879648-22-3 65%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 95; for 2 h;Inert atmosphere;
Steps:
257.1 Step 1: 4,4,5,5-Tetramethyl-2-(2-(methylsulfonyl)phenyl)- 1,3 ,2-dioxaborolane
Step 1: 4,4,5,5-Tetramethyl-2-(2-(methylsulfonyl)phenyl)- 1,3 ,2-dioxaborolane A suspension of 1-bromo-2-(methylsulfonyl)benzene (800 mg, 3.40 mmol),bis(pinacolato)diboron (1040 mg, 4.08 mmol) and potassium acetate (668 mg, 6.81 mmol) in dioxane (4 ml) was degassed with bubbling nitrogen. PdC12(dppf)-CH2C12 adduct (139 mg, 0.170 mmol) was added, and the reaction was heated to 95 °C for 2 h. The reaction was diluted with EtOAc (30 mL) and filtered through Celite. The organic layer was washed with brine and dried with sodium sulfate. The solvent was evaporated, and the residue was purified by column chromatography on silica gel (40 g column,gradient from 0% to 40% EtOAc/hexanes) to give the title compound (626 mg, 65 %) as a colorless oil. ‘H NMR (400MHz, CDC13) ? 8.03 (dd, J=7.6, 1.2 Hz, 1H), 7.72-7.67 (m, 1H), 7.61 (dtd, J=18.6, 7.4, 1.5 Hz, 2H), 5.32 (s, 1H), 3.24 (s, 3H), 1.43 (s, 12H); LCMS (M+H) = 283.2; HPLC RT = 0.89 mm (Column: BEH C18 2.1 x 50 mm; Mobile PhaseA: Water with 0.05% TFA; Mobile Phase B: Acetonitrile with 0.05% TFA; Gradient: 2-98% B over 1.6 mm; Flow: 0.8 mL/min).
References:
WO2015/100282,2015,A1 Location in patent:Page/Page column 282; 283