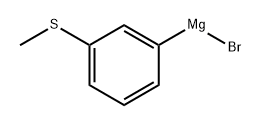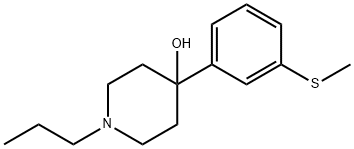
4-Hydroxy-4-(3-methylsulfanylphenyl)-1-propylpiperidine synthesis
- Product Name:4-Hydroxy-4-(3-methylsulfanylphenyl)-1-propylpiperidine
- CAS Number:882737-40-8
- Molecular formula:C15H23NOS
- Molecular Weight:265.41
23133-37-1
183 suppliers
$20.00/5g
33733-73-2
150 suppliers
$5.00/1g

882737-40-8
8 suppliers
inquiry
Yield:882737-40-8 70%
Reaction Conditions:
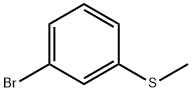
Stage #1: 3-bromo-1-(methylthio)benzenewith n-hexyllithium in tetrahydrofuran;hexane at 1 - 2;
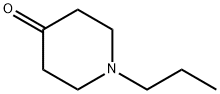
Stage #2: 1-propyl-4-piperidone in tetrahydrofuran;hexane;Temperature;
Steps:
3 Compound 9 was prepared following the synthesis hereinbelow:
A solution of 50% w/w 3-bromothioanisole (3-BTA) in THF extra dry ( 30 g/min, Tj 5 s, Tr = (-30) - 10°C, P=l.5-15 bar). The lithiatedCompound 9 solution is quenched by flowing into a reactor containing 5 Vol of water at 5°C (Tr < 30°C), forming a solid Compound 9 FB. A washing cycle is done every 40-45 mm. The washing cycle includes 1-1.5 mm solvent flowing wash (THF/n-hexane), then washing with water for 8-10 mmand finally washing again with solvents (THF/n-hexane) for 1-1.5 mi When the flow reaction isover, 5 Vol of toluene are added into the reactor. The reaction mixture is warmed up to 40°C in orderto dissolve the solid Compound 9 FB into the organic phase. The two phases are mixed together at40°C (Tr = 35-50°C, pH=l2-14) for 30 mm, the mixing is stopped to allow phase separation and after20 mm the lower aqueous cloudy phase is removed (first extraction). Four Vol of fresh water areadded and mixed for 20 mm, keeping the temperature at 40°C (Tr = 35-50°C, pH=ll-13). The mixing is stopped to allow the phases to separate, giving a clear aqueous phase which is removed after 15 mm (second extraction). Four (4) Vol of fresh water are added and mixed for 20 mm at 40°C (Tr = 35- 50°C, pHl 0). The mixing is stopped for 15 mm and the lower, clear aqueous phase is removed (last extraction). The reaction mixture is cooled down to Tr<1 5°C and distilled in vacuum in two stages. Inthe first stage, when the mixture is foaming, the pressure is reduced to P<80 mbar, the Tj is carefully warmed to 25°C and the stirring is on high speed to break bubbles. After most of the T1{F has been distilled (1/2 Vol in the distillate), the Tj is warmed up to 40-60°C and the P<90 mbar until 2-3 Vol remain in the reactor. One (1) Vol of n-heptane is added at 35-45°C and the slurry that was formed is dissolved by warming up to 50-60°C. When a clear solution is obtained the mixture is cooled down to40-50°C for crystallization. The mixture is mixed at 40-50°C for not less than (NLT) 4 h (breeding time). The slurry is cooled down over 4 h to -5°C and mixed for not less than 4 h. Three (3) Vol of nheptane are added to the slurry and mixed for an additional 1 h. The solid is filtered, washed with 2 Vol n-heptane and dried under vacuum (P<50 mbar) at 40°C to constant weight. Dried Compound 9 FB is obtained as a yellowish to white solid, with 50%-70% yield.
References:
WO2017/15609,2017,A1 Location in patent:Page/Page column 21; 22; 23; 24; 25; 26