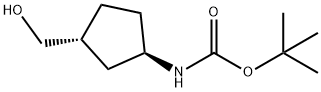
CarbaMic acid, [(1R,3R)-3-(hydroxyMethyl)cyclopentyl]-, 1,1-diMethylethyl ester (9CI) synthesis
- Product Name:CarbaMic acid, [(1R,3R)-3-(hydroxyMethyl)cyclopentyl]-, 1,1-diMethylethyl ester (9CI)
- CAS Number:884006-56-8
- Molecular formula:C11H21NO3
- Molecular Weight:215.29
![methyl (1R,3R)-3-{[(tert-butoxy)carbonyl]amino}cyclopentane-1-carboxylate](/CAS/20180527/GIF/489446-72-2.gif)
489446-72-2
27 suppliers
inquiry
![CarbaMic acid, [(1R,3R)-3-(hydroxyMethyl)cyclopentyl]-, 1,1-diMethylethyl ester (9CI)](/CAS/20150408/GIF/884006-56-8.gif)
884006-56-8
18 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: (1R,3R)-3-tert-butoxycarbonylaminocyclopentanecarboxylic acid methyl esterwith lithium borohydride in tetrahydrofuran at 0 - 20; for 12.5 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 0;
Steps:
3
To a stirred and cooled (0 0C) solution of Step 2 intermediate (8.0 g, 34.89 mmol) in dry THF (100 ml) was added lithium borohydride (2.64 g, 69.8 mmol) in portions over a period of 30 min. The mixture was further stirred at RT for 12 h. Excess lithium borohydride was quenched with IiVHCl at 0 °C. The mixture was extracted with dichloromethane (2 x 100 ml) and the combined extracts were washed with water (200 ml), brine (100 ml) and dried (Na2SO4). The solvent was evaporated under reduced pressure to give 4.3 g of the product as a white solid; IR (KBr) 3338, 2973, 1688, 1526, 1391, 1366, 1300, 1250, 1171, 1047 cm"1; 1H NMR (CDCl3, 300 MHz) δ 1.27-1.47 (m, 2H), 1.44 (s, 9H), 1.51-1.65 (m, IH), 1.67-1.91 (m, 2H), 2.00-2.05 (m, IH), 2.18-2.30 (m, IH), 3.51 (d, J= 7.2 Hz, 2H), 3.98 (brs, IH), 4.58 (brs, IH).
References:
WO2006/40625,2006,A1 Location in patent:Page/Page column 43