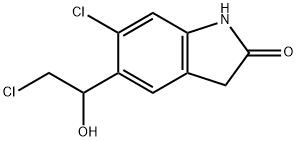
Ziprasidone IMpurity (6-Chloro-5-(2-Chloro-1-Hydroxy-Ethyl)-1,3-Dihydro-Indol-2-One) synthesis
- Product Name:Ziprasidone IMpurity (6-Chloro-5-(2-Chloro-1-Hydroxy-Ethyl)-1,3-Dihydro-Indol-2-One)
- CAS Number:884305-06-0
- Molecular formula:C10H9Cl2NO2
- Molecular Weight:246.09
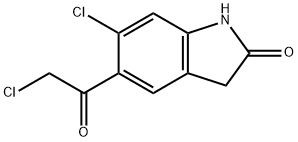
118307-04-3
154 suppliers
$12.00/250mg

884305-06-0
29 suppliers
inquiry
Yield:884305-06-0 88%
Reaction Conditions:
Stage #1: 6-chloro-5-(2-chloroacetyl) indolin-2-onewith sodium tetrahydroborate in ethanol at 15 - 20; for 15 h;
Stage #2: with acetic acid in ethanol at 85; for 2 h;
Steps:
1
Example 1. 6-Chloro-5-(2-chloro-1-hydroxy-ethyl)-1,3-dihydro-indol-2-one. (V); A reactor is loaded with 200 g (0.82 mol) of 6-chloro-5-(2-chloroacetyl)-1,3-dihydro-indol-2-one, in 1 L of ethanol. The reaction mixture is added with 15.5 g (0.41 mol) of sodium borohydride in portions, under strong stirring, at room temperature. The temperature is kept at 15-20°C during the addition, after that the mixture is left under stirring for about 15 h at room temperature. The mixture is then added with 250 ml of glacial acetic acid and refluxed (approx. 85°C) for 1 hr 30 min, then 200 ml of acetic acid are added and the mixture is refluxed a further 30 min, then cooled to 20°C. The suspension is filtered and washed with ethanol and heptane. The solid product is dried under vacuum at a temperature of 60°C, to obtain 177 g (88% molar yield).
References:
EP1787990,2007,A2 Location in patent:Page/Page column title page; 4

118307-04-3
154 suppliers
$12.00/250mg

884305-06-0
29 suppliers
inquiry
118289-55-7
271 suppliers
$7.00/250mg
56341-37-8
402 suppliers
$15.00/5g

884305-06-0
29 suppliers
inquiry

56341-37-8
402 suppliers
$15.00/5g

884305-06-0
29 suppliers
inquiry

118289-55-7
271 suppliers
$7.00/250mg