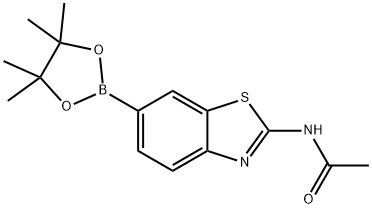
N-(6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazol-2-yl)acetamide synthesis
- Product Name:N-(6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazol-2-yl)acetamide
- CAS Number:885069-14-7
- Molecular formula:C15H19BN2O3S
- Molecular Weight:318.2
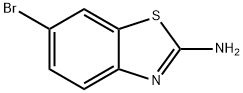
![N-(6-bromobenzo[d]thiazol-2-yl)acetamide](/CAS/GIF/16628-26-5.gif)
16628-26-5

73183-34-3
![N-(6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazol-2-yl)acetamide](/CAS/GIF/885069-14-7.gif)
885069-14-7
General procedure for the synthesis of 2-acetamido benzothiazole-6-boronic acid from 6-bromo-2-acetamidobenzothiazole and pinacol ester of bis(pinacolato): N-(6-bromobenzo[d]thiazol-2-yl)acetamide (2.10 g, 7.75 mmol), bis(pinacolato)diboron (3.00 g, 11.8 mmol), Pd(dppf)Cl2 ( 560 mg, 0.77 mmol) and KOAc (3.00 g, 30.6 mmol) were dissolved in DMSO (50 mL) and the reaction was stirred at 90 °C for 8 h under N2 atmosphere. After completion of the reaction, it was cooled to room temperature and the reaction mixture was filtered. The filtrate was diluted with ethyl acetate (200 mL), washed sequentially with brine (20 mL x 3) and the organic phase was dried over anhydrous Na2SO4 and concentrated. The resulting residue was washed with petroleum ether (50 mL) to give the light brown solid product 2-acetamido benzothiazole-6-boronic acid (2.40 g, 97% yield).
![N-(6-bromobenzo[d]thiazol-2-yl)acetamide](/CAS/GIF/16628-26-5.gif)
16628-26-5
14 suppliers
$503.86/5MG

73183-34-3
587 suppliers
$6.00/5g
![N-(6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazol-2-yl)acetamide](/CAS/GIF/885069-14-7.gif)
885069-14-7
21 suppliers
inquiry
Yield:885069-14-7 97%
Reaction Conditions:
with palladium (II) [1,1'-bis(diphenylphosphanyl)ferrocene] dichloride;anhydrous potassium acetate in (methylsulfinyl)methane at 90; for 8 h;Inert atmosphere;Miyaura Borylation Reaction;
Steps:
N-(6-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazol-2-yl)acetamide (6)
A mixture of N-(6-bromobenzo[d]thiazol-2-yl)acetamide (2.10 g, 7.75 mmol), bis(pinacolato)diboron (3.00 g, 11.8 mmol), Pd(dppf)Cl2 (560 mg, 0.77 mmol), KOAc (3.00 g, 30.6 mmol) and DMSO (50 mL) was stirred at 90 °C under N2 atmosphere for 8 h. After cooling to room temperature, the mixture was filtered. The filtrate was diluted with ethyl acetate (200 mL), washed with brine (20 mL * 3), dried overNa2SO4, and concentrated. The resulting residue was washed with petroleum ether (50 mL) to give a pale brown solid (2.40 g, 97%).
References:
Yang, Zhaohui;Ma, Haikuo;Sun, Zhijian;Luo, Lusong;Tian, Sheng;Zheng, Jiyue;Zhang, Xiaohu [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 17,art. no. 22839,p. 3665 - 3670] Location in patent:supporting information
15864-32-1
237 suppliers
$6.00/1g
![N-(6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazol-2-yl)acetamide](/CAS/GIF/885069-14-7.gif)
885069-14-7
21 suppliers
inquiry

106-40-1
484 suppliers
$12.00/5g
![N-(6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazol-2-yl)acetamide](/CAS/GIF/885069-14-7.gif)
885069-14-7
21 suppliers
inquiry

15864-32-1
237 suppliers
$6.00/1g

73183-34-3
587 suppliers
$6.00/5g
![N-(6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]thiazol-2-yl)acetamide](/CAS/GIF/885069-14-7.gif)
885069-14-7
21 suppliers
inquiry