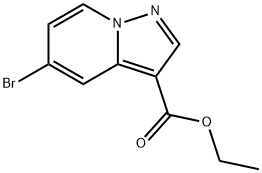
5-BROMO-PYRAZOLO[1,5-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:5-BROMO-PYRAZOLO[1,5-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:885276-93-7
- Molecular formula:C10H9BrN2O2
- Molecular Weight:269.09
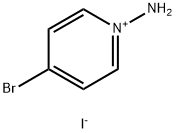
1533440-88-8
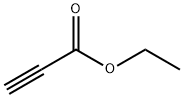
623-47-2
![5-BROMO-PYRAZOLO[1,5-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/885276-93-7.gif)
885276-93-7
General procedure for the synthesis of ethyl 5-bromopyrazolo[1,5-a]pyridine-3-carboxylate from 1-amino-4-bromopyridinium iodide and ethyl propargylate: To a 250 mL three-necked round-bottomed flask was added 1-amino-4-bromopyridinium iodide (Compound 64.2, 15 g, ca. 50% purity, 24.9 mmol) in N,N-dimethylformamide (80 mL) Solution. Potassium carbonate (10.6 g, 76.7 mmol) was added batchwise, followed by ethyl propargylate (11.7 mL, 115 mmol) dropwise over 10 min. The reaction mixture was stirred overnight at room temperature and then diluted with ethyl acetate (300 mL) and water (100 mL). Insoluble solids were removed by filtration, and the organic layer was washed with saturated saline (3 x 50 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography using ethyl acetate/petroleum ether (1:10) as eluent to afford ethyl 5-bromopyrazolo[1,5-a]pyridine-3-carboxylate as a brown solid (300 mg, 6% yield).

1533440-88-8
5 suppliers
inquiry

623-47-2
352 suppliers
$10.00/1g
![5-BROMO-PYRAZOLO[1,5-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/885276-93-7.gif)
885276-93-7
125 suppliers
$36.00/100mg
Yield:885276-93-7 300 mg
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide; for 0.166667 h;
Steps:
1 Compound 64.3. Ethyl 5-bromopyrazolo[l,5-a]pyridine-3-carboxylate
Compound 64.3. Ethyl 5-bromopyrazolo[l,5-a]pyridine-3-carboxylate. Into a 250-mL three neck round-bottom flask, was placed a solution of l-amino-4-bromopyridin-l- ium iodide (compound 64.2, 15 g, -50% purity, 24.9 mmol) in N.N-dimethylformamide (80 mL). Potassium carbonate (10.6 g, 76.7 mmol) was added portion-wise followed by the addition of ethyl propiolate (1 1.7 mL, 1 15 mmol) drop-wise over 10 min. The resulting mixture was stirred overnight at room temperature, then diluted with EtOAc (300 mL) and water (100 mL). The solids were removed by filtration and the organic layer was washed with brine (3 x 50 mL), dried (Na2S04), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography with ethyl acetate/petroleum ether (1 : 10) as the eluent to yield the title compound as a brown solid (300 mg, 6%).
References:
WO2014/8197,2014,A1 Location in patent:Page/Page column 135
1120-87-2
166 suppliers
inquiry

623-47-2
352 suppliers
$10.00/1g
![5-BROMO-PYRAZOLO[1,5-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/885276-93-7.gif)
885276-93-7
125 suppliers
$36.00/100mg
![ethyl 5-((tert-butoxycarbonyl)aMino)pyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/GIF/1101120-33-5.gif)
1101120-33-5
47 suppliers
$45.00/10mg
![5-BROMO-PYRAZOLO[1,5-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/885276-93-7.gif)
885276-93-7
125 suppliers
$36.00/100mg
1060814-91-6
52 suppliers
$65.00/10mg
![5-BROMO-PYRAZOLO[1,5-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/885276-93-7.gif)
885276-93-7
125 suppliers
$36.00/100mg
98400-69-2
172 suppliers
$6.00/1g
![5-BROMO-PYRAZOLO[1,5-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/885276-93-7.gif)
885276-93-7
125 suppliers
$36.00/100mg