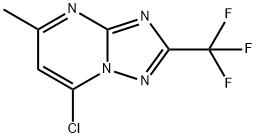
7-chloro-5-Methyl-2-(trifluoroMethyl)-[1,2,4]triazolo[1,5-a]pyriMidine synthesis
- Product Name:7-chloro-5-Methyl-2-(trifluoroMethyl)-[1,2,4]triazolo[1,5-a]pyriMidine
- CAS Number:885461-50-7
- Molecular formula:C7H4ClF3N4
- Molecular Weight:236.58
![5-methyl-2-(trifluoromethyl)[1,2,4]triazolo[1,5-a]pyrimidin-7-ol](/CAS/20180703/GIF/116529-17-0.gif)
116529-17-0
3 suppliers
inquiry
![7-chloro-5-Methyl-2-(trifluoroMethyl)-[1,2,4]triazolo[1,5-a]pyriMidine](/CAS/20150408/GIF/885461-50-7.gif)
885461-50-7
22 suppliers
$112.00/100mg
Yield:885461-50-7 53.1%
Reaction Conditions:
with trichlorophosphate for 2 h;Reflux;
Steps:
3.3 Step 3:
[0098] Step 3: Phosphoryl chloride (5 mL) was added to 8 (0.3 g, 13.7 mmol) and the reaction mixture was heated toreflux for 2 h. The reaction mixture was cooled to 25°C and phosphoryl chloride was removed under reduced pressure.Crude product was quenched with crushed ice and neutralized with 10% NaHCO3 solution and extracted with ethyl acetate (2 x 5 mL). The combined organic layers were washed with brine solution, dried over Na2SO4 and concentratedunder reduced pressure. Crude product was purified by column chromatography using 15-25% pet. ether and ethylacetate to yield compound 5c, 7-Chloro-5-methyl-2-(trifluoromethyl) -[1,2,4]triazolo[1,5-a] pyrimidine (0.17 g, 53.1%).1H NMR (400 MHz, CDCl3): δ 7.27 (s, 1H), 2.78 (s, 3H).
References:
EP3072894,2016,A1 Location in patent:Paragraph 0098