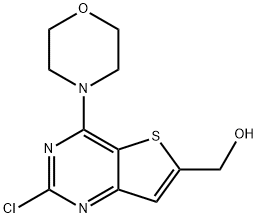
2-Chloro-4-(4-Morpholinyl)thieno[3,2-d]pyriMidine-6-Methanol synthesis
- Product Name:2-Chloro-4-(4-Morpholinyl)thieno[3,2-d]pyriMidine-6-Methanol
- CAS Number:885698-97-5
- Molecular formula:C11H12ClN3O2S
- Molecular Weight:285.75
124773-65-5
0 suppliers
inquiry
![2-Chloro-4-(4-Morpholinyl)thieno[3,2-d]pyriMidine-6-Methanol](/CAS/GIF/885698-97-5.gif)
885698-97-5
15 suppliers
inquiry
Yield:885698-97-5 60%
Reaction Conditions:
with sodium tetrahydroborate in methanol;water;ethyl acetate;
Steps:
116.a Step 2a:
Step 2a:
(2-chloro-4-morpholinothieno[3,2-d]pyrimidin-6-yl)methanol (Intermediate 2a)
To a solution of compound 1b (5 g, 17.6 mmol) in MeOH (50 mL) was added NaBH4 (0.98 g, 26.4 mmol) portion wise at 0° C. and stirred for 5 h at RT.
After the completion of reaction (monitored by TLC), the volatiles were removed under reduced pressure, residue dissolved in water and extracted with DCM (3*75 mL).
The combined organic phases were washed with water, dried over anhydrous Na2SO4 and concentrated in vacuo to afford intermediate 6a (3 g, 60%) as a light yellow solid. TLC: 80% EtOAc/Hexane (Rf: 0.3); 1H-NMR (CDCl3, 200 MHz): δ 7.21 (s, 1H), 4.98 (s, 2H), 4.0 (t, J=4.2 Hz, 4H), 3.83 (t, J=4.8 Hz, 4H); Mass: 286 [M++1]
References:
US2011/269244,2011,A1 Location in patent:Page/Page column