
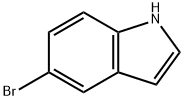
TERT-BUTYL 4-(5-BROMO-1H-INDOL-3-YL)-3,6-DIHYDRO-1(2H)-PYRIDINECARBOXYLATE synthesis
- Product Name:TERT-BUTYL 4-(5-BROMO-1H-INDOL-3-YL)-3,6-DIHYDRO-1(2H)-PYRIDINECARBOXYLATE
- CAS Number:886361-90-6
- Molecular formula:C18H21BrN2O2
- Molecular Weight:377.28
10075-50-0
689 suppliers
$5.00/10g

79099-07-3
0 suppliers
$5.00/5g

886361-90-6
21 suppliers
$192.00/1g
Yield:886361-90-6 86%
Reaction Conditions:
with sodium methylate in methanol at 80; for 48 h;
Steps:
9.A
To a solution of commercially available 5-bromoindole (3.5 g, 17.9 mmol) in methanol (50 mL) was added 1-Boc-4-piperidone (5.1 g, 25.6 mmol) and a solution of 25% sodium methoxide in methanol (8 mL, 37 mmol) and the reaction mixture was heated at 80° C. for 2 days. The reaction mixture was filtered off. The solid was washed twice with ethyl acetate and dried under vacuum to give the title compound (3 g). The filtrate was diluted with ethyl acetate (250 mL) and washed with water, brine and dried over Na2SO4. The solvent was removed under reduced pressure to yield the crude product, which was purified on a silica gel column to afford additional title compound (2.9 g). The combined yield was 5.9 g, 86%.1H-NMR (400 MHz, CDCl3): δ=1.52 (s, 9H), 2.55 (m, 2H), 3.69 (t, 2H), 4.16 (s, 2H), 6.12 (s, 1H), 7.19 (s, 1H), 7.26-7.33 (m, 2H), 8.01 (s, 1H), 8.29 (br-s, 1H)
References:
US2011/280808,2011,A1 Location in patent:Page/Page column 44