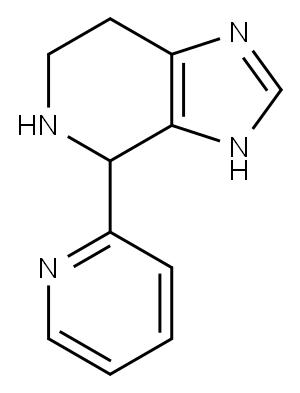
4-pyridin-2-yl-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine(SALTDATA: H2O) synthesis
- Product Name:4-pyridin-2-yl-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine(SALTDATA: H2O)
- CAS Number:887405-39-2
- Molecular formula:C11H12N4
- Molecular Weight:200.24
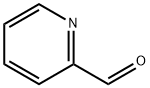
1121-60-4
500 suppliers
$10.60/10gm:
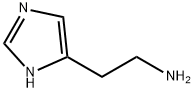
51-45-6
253 suppliers
$9.00/50mg
![4-pyridin-2-yl-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine(SALTDATA: H2O)](/CAS/GIF/887405-39-2.gif)
887405-39-2
15 suppliers
$45.00/10mg
Yield:887405-39-2 20%
Reaction Conditions:
with potassium hydroxide in ethanol; for 48 h;Reflux;
Steps:
21
A mixture of histamine (0.1 1 mmol), potassium hydroxide (0.33 mmol) and 2-formylpyridine (0.13 mmol) were refluxed in ethanol for 48 h, after which the solvent was evaporated and the products chromatographed on silica (1% methanol/DCM to 12% methanol saturated with ammonia) to yield 4-(pyrid-2-yl)-4,5,6,7-tetrahydro-lH-imidazo[4,5-c]pyridine (18.8 mg; 20%) as a white solid. 1H NMR (400 MHz, CD3OD) δ 8.49 (d, J= 5.4 Hz, IH), 7.87 (t, J = 5.4 Hz, IH), 7.62 (s, IH), 7.54-7.40 (m, 2H),3.3-2.7 (m, 4H); 13C NMR (100 MHz, CD3OD) δ 160.0, 149.4, 138.8, 135.7, 131.4 (b), 128.3 Qo), 123.0, 58.2, 49.5, 41.0, 22.6;0 m/z 201 (100; M+H+).4-(Pyrid-2-yl)-4,5,6,7-tetrahydro-lH-imidazo[4,5-c]pyridine (0.05 mmol) and IBX (0.075 mmol) were dissolved in DMSO (300 μL) and heated to 45 °C for 7 hours. Flash chromatography on silica gel (1% methanol/DCM to 12% methanol saturated with ammonia) yielded 4-(pyrid-2-yl)- lH-imidazo[4,5-c]pyridine (9 mg; 90%) as a paleS yellow solid 1H NMR (400 MHz, CD3OD) δ 8.74 (m, 2H), 8.55 (d, J = 5.4 Hz, IH), 8.32 (s, IH), 7.92 (t, IH), 7.85 (d, IH), 7.38 (dd, IH); m/z 197 (100; M+H+)
References:
WO2009/152584,2009,A1 Location in patent:Page/Page column 31