
Methyl 1-(2-chloropyrimidin-4-yl)piperidine-4-carboxylate synthesis
- Product Name:Methyl 1-(2-chloropyrimidin-4-yl)piperidine-4-carboxylate
- CAS Number:889126-33-4
- Molecular formula:C11H14ClN3O2
- Molecular Weight:255.7
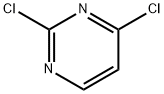
3934-20-1
725 suppliers
$9.00/1g
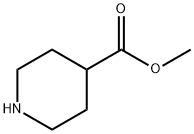
2971-79-1
272 suppliers
$10.00/10g

889126-33-4
27 suppliers
$100.00/1 g
Yield:-
Reaction Conditions:
with triethylamine in methanol at 80; for 24 h;
Steps:
8.A
1.43g Methyl piperidine-4-carboxylate (lOmmol) was mixed with 2.01g 2,4- dichloropyrimidine (13.5mmol) and 3.04g triethylamine in 5OmL methanol. The reaction was heated to 8O0C for 24hr. The volatiles were removed in vacuo and the crude residue was purified on silica gel eluted with a linear gradient between 40-60% EtOAc/hexanes. This provided the title compound. 1H- NMR (CDCl3): δ 1.72-1.82 (m, 2H), 2.00-2.07 (m, 2H), 2.65 (tt, IH, J= 4, 1 IHz), 3.14 (ddd, 2H, J= 3,11 , 14Hz), 3.74 (s, 3H), 4.22 (bs, 2H), 6.42 (d, IH, J= 7Hz), 8.05 (d, IH, J= 7Hz) ppm.
References:
MERCK & CO., INC. WO2006/73589, 2006, A2 Location in patent:Page/Page column 28