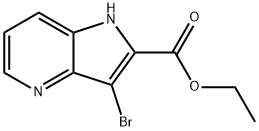
3-broMo-1H-pyrrolo[3,2-b]pyridine-2-carboxylic acid synthesis
- Product Name:3-broMo-1H-pyrrolo[3,2-b]pyridine-2-carboxylic acid
- CAS Number:889658-85-9
- Molecular formula:C10H9BrN2O2
- Molecular Weight:269.09
![1H-Pyrrolo[3,2-b]pyridine-2-carboxylic acid ethyl ester](/CAS2/GIF/17288-32-3.gif)
17288-32-3
117 suppliers
$26.00/250mg
![3-broMo-1H-pyrrolo[3,2-b]pyridine-2-carboxylic
acid](/CAS/GIF/889658-85-9.gif)
889658-85-9
33 suppliers
$100.00/100mg
Yield:889658-85-9 61%
Reaction Conditions:
with N-Bromosuccinimide in dichloromethane at 0 - 20; for 16 h;
Steps:
1.1 Step 1. Synthesis of ethyl 3-bromo- I H -p yrrolo[3 , 2-b]pyridine-2-carboxylate (Cl).
N-Bromosuccinimide (15.4 g, 86.5 mmcl) was added to a 0 °C solution of ethyl 1 H-pyrrolo[3,2-b]pyridine-2-carboxylate (15.0 g, 78.9 mmol) in dichloromethane (150 mL), and the reaction mixture was stirred at room temperature for 16 hours. Afteraddition of dichloromethane (150 mL) and water (200 mL), the aqueous layer was extracted with dichloromethane (3 x 150 mL). The combined organic layers were washed with saturated aqueous sodium chloride solution (5 x 50 mL), dried over sodium sulfate, filtered, and concentrated in vacuo. Silica gel chromatography (Gradient: 0% to 50% ethyl acetate in petroleum ether) afforded the product as a yellowsolid. Yield: 13 g, 48 mmol, 61%. NMR (400 MHz, CDCI3) ? 9.94 (br s, 1 H), 8.65 (dd, J=4.5, 1.1 Hz, 1H), 7.78 (dd, J=8.4, 1.0 Hz, 1H), 7.31 (dd, J=8.4, 4.5 Hz, 1H), 4.49 (q, J=7.1 Hz, 2H), 1.45 (t, J=7.1 Hz, 3H).
References:
WO2016/203347,2016,A1 Location in patent:Page/Page column 86
18699-87-1
250 suppliers
$8.00/1g
![3-broMo-1H-pyrrolo[3,2-b]pyridine-2-carboxylic
acid](/CAS/GIF/889658-85-9.gif)
889658-85-9
33 suppliers
$100.00/100mg