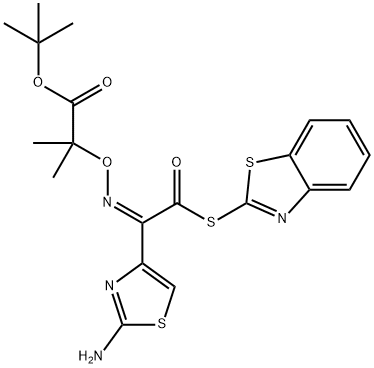
2-Mercaptobenzothiazolyl-(Z)-(2-aminothiazol-4-yl)-2-(tert-butoxycarbonyl) isopropoxyiminoacetate synthesis
- Product Name:2-Mercaptobenzothiazolyl-(Z)-(2-aminothiazol-4-yl)-2-(tert-butoxycarbonyl) isopropoxyiminoacetate
- CAS Number:89604-92-2
- Molecular formula:C20H22N4O4S3
- Molecular Weight:478.61
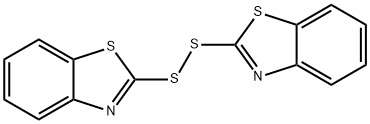
120-78-5
![(Z)-2-Amino-alpha-[1-(tert-butoxycarbonyl)]-1-methylethoxyimino-4-thiazolacetic acid](/CAS/GIF/86299-47-0.gif)
86299-47-0

89604-92-2
Synthesis of (Z)-2-(((1-(2-aminothiazol-4-yl)-2-((1-(tert-butoxy)-2-methyl-1-oxopropan-2-yl)oxy)imino)acetic acid from dibenzothiazole disulfide and (Z)-2-((2-(2-aminothiazol-4-yl)-2-(benzo[d]thiazol-2-ylthio)-2-oxoethylidene)amino)oxy) The general procedure for tert-butyl -2-methylpropanoate was as follows: 35 g of (Z)-2-(2-aminothiazol-4-yl)-2-(((1-(tert-butoxy)-2-methyl-1-oxopropan-2- yl)oxy)imino)acetic acid and 48 g of dibenzothiazole disulfide were added to a solvent mixture of dichloromethane and acetonitrile at 21 °C. (the total mass of the solvent mixture was 250 g, the density of 1.0 g/cm3). After stirring for 10 minutes, 1.0 mL of pyridine was added. At 22°C, 8.0 mL of triethylamine was slowly added. After addition, the reaction temperature was raised to 30°C and kept at this temperature for 50 minutes. Subsequently, the reaction mixture was cooled to 21 °C, and 28 mL of triethyl phosphite was slowly added dropwise at this temperature, with the dropwise addition process controlled to be completed within 2.5 hours. After the dropwise addition was completed, the reaction solution was continued at 21±1 °C for 3.0 hours. After that, the reaction mixture was cooled to 3°C and kept at this temperature for 30 minutes. Upon completion of the reaction, filtration was carried out, the solid product was collected and dried at 60 °C to afford the target product (Z)-tert-butyl 2-(((1-(2-aminothiazol-4-yl)-2-(benzo[d]thiazol-2-ylsulfanyl)-2-oxoethylidene)amino)oxy)-2-methylpropanoate in 92.6% yield and 99.3% purity.

120-78-5
508 suppliers
$5.00/10g
![(Z)-2-Amino-alpha-[1-(tert-butoxycarbonyl)]-1-methylethoxyimino-4-thiazolacetic acid](/CAS/GIF/86299-47-0.gif)
86299-47-0
174 suppliers
$19.00/1g

89604-92-2
175 suppliers
$49.00/1g
Yield:89604-92-2 92.6%
Reaction Conditions:
Stage #1:di(benzothiazol-2-yl)disulfide;(Z)-2-(2-aminothiazol-4-yl)-2-(1-tert-butoxycarbonyl-1-methylethoxy)iminoacetic acid with pyridine;triethylamine in acetonitrile at 21 - 30; for 0.833333 h;
Stage #2: with triethyl phosphite in acetonitrile at 21; for 5.5 h;Temperature;
Steps:
2 Example 2
At 21 ° C,35g cefotaxime side chain acid and 48gDMWas added to a mixture of dichloro and acetonitrile (the mass of the mixture was 250 g and the density was 1.0 g / cm 3)After stirring for 10 min,1.0 mL of pyridine was added,At 22 ° C, triethylamine (8.0 mL)After the addition was completed, the temperature was raised to 30 ° C., the reaction was incubated for 50 minutes, cooled,28 ml of triethyl phosphite was added dropwise at 21 ° C,Drop when the process of sharing 2.5h,After the addition was completed, the reaction was incubated at 21 ± 1 ° C for 3.0 h,Then cooled to 3 incubated reaction 30min, suction filtration,And dried at 60 to ceftazidime side chain acid active ester, the yield was 92.6%The content is 99.3%.
References:
Zibo Xinquan Pharmaceutical Services Co., Ltd.;Zhang Yanhong;Liu Chengxue;Zhang Liming CN106699683, 2017, A Location in patent:Paragraph 0028-0033

120-78-5
508 suppliers
$5.00/10g
![(Z)-2-Amino-alpha-[1-(tert-butoxycarbonyl)]-1-methylethoxyimino-4-thiazolacetic acid](/CAS/GIF/86299-47-0.gif)
86299-47-0
174 suppliers
$19.00/1g

89604-92-2
175 suppliers
$49.00/1g
149-30-4
683 suppliers
$5.00/25g