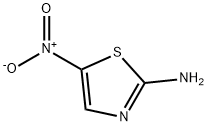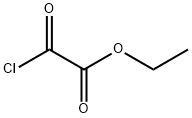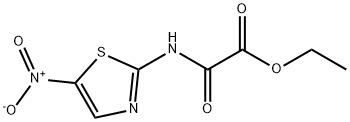
Ethyl [(5-nitro-1,3-thiazol-2-yl)amino](oxo)acetate synthesis
- Product Name:Ethyl [(5-nitro-1,3-thiazol-2-yl)amino](oxo)acetate
- CAS Number:89792-36-9
- Molecular formula:C7H7N3O5S
- Molecular Weight:245.21
Yield:89792-36-9 90%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 6 h;
Steps:
4 Synthesis of ethyl [(5-nitro-1,3-thiazol-2-yl)amino](oxo)acetate (14)
To a solution of 2-amino-5-nitro-1,3-thiazole (0.0015 mol) in dichloromethane, was added triethylamine (1.2 equiv). The reaction mixture was stirred at 5 °C for 15 min. After that, a solution of ethyl chlorooxoacetate (0.0018 mol, 1.2 equiv) was added droopingly. The reaction mixture was stirred at room temperature for 6 h. After complete conversion as indicated by TLC, the solvent was removed in vacuo, the residue was neutralized with saturated NaHCO3 solution, and the aqueous layer was extracted with ethyl acetate (3 x 15 mL), washed with water (3 * 20 mL), and dried over anhydrous Na2SO4. The solvent was evaporated in vacuo and the precipitated solids were recrystallized from a mixture of acetonitrile/methanol. Yield: 90%, mp: 252-255 °C. 1H NMR (400 MHz, DMSO-d6) δ: 8.67 (1H, s, H-4), 4.32 (2H, q, O-CH2) 1.31 (3H, t, CH3) ppm; 13C NMR (100 MHz, DMSO-d6) δ: 161.7 (C-2), 158.4 (CO-OR), 157.7 (RNH-CO), 143.3 (C-5), 142.7 (C-4), 63.4 (O-CH2), 14.1 (CH3) ppm; MS (FAB+) m/z 246 (M+H+). Anal. Calcd for C7H7N3O5S: C, 34.29; H, 2.88; N, 17.14. Found: C, 34.19; H, 2.83; N, 17.09.
References:
Nava-Zuazo, Carlos;Chávez-Silva, Fabiola;Moo-Puc, Rosa;Chan-Bacab, Manuel Jesús;Ortega-Morales, Benjamín Otto;Moreno-Díaz, Hermenegilda;Díaz-Couti?o, Daniel;Hernández-Nú?ez, Emanuel;Navarrete-Vázquez, Gabriel [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 5,p. 1626 - 1633]