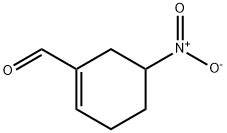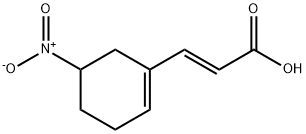
2-Propenoic acid, 3-(5-nitro-1-cyclohexen-1-yl)-, (2E)- synthesis
- Product Name:2-Propenoic acid, 3-(5-nitro-1-cyclohexen-1-yl)-, (2E)-
- CAS Number:899809-64-4
- Molecular formula:C9H11NO4
- Molecular Weight:197.19
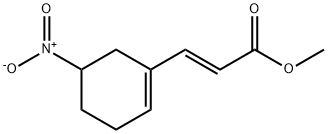
900186-90-5

899809-64-4
General procedure for the synthesis of (E)-3-(5-nitro-1-cyclohexen-1-yl)acrylic acid from methyl (E)-3-(5-nitro-1-cyclohexen-1-yl)acrylate: to a 1 L methanol solution at 0 °C was added methyl (E)-3-(5-nitro-1-cyclohexen-1-yl)acrylate (67 g, 432 mmol) and Wittig reagent (144.4 g, 432 mmol). The reaction mixture was stirred at 0°C for 3 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was extracted twice with methyl tert-butyl ether (MeOBu-t). The organic phases were combined, filtered to remove insoluble solids, washed with saturated saline and concentrated. The residue was purified by silica gel column chromatography, and 9.2 g of the cis product and 55.1 g (60.4% yield) of the trans product were isolated using hexane/ethyl acetate (10:1, v/v) as eluent. The product structures were confirmed by 1H NMR (CDCl3): δ 7.31 (d, J = 11.3 Hz, 1H), 6.18 (m, 1H), 5.84 (d, J = 15.9 Hz, 1H), 4.74-4.68 (m, 1H), 3.76 (s, 3H), 2.81-2.74 (m, 2H), 2.50-2.04 (m, 4H) . Subsequently, the trans product (2.1 g) was dissolved in a solvent mixture of methanol (9.6 ml) and water (2.4 ml). A 50% aqueous sodium hydroxide solution (0.96 ml) was slowly added dropwise to the solution at about 5°C. After the dropwise addition, the reaction mixture was gradually warmed up to room temperature and stirred for 24 hours. After completion of the reaction, it was neutralized with acetic acid (HOAc) to pH 4-5 and methanol was removed by distillation under reduced pressure. The residue was extracted with ethyl acetate (3 x 50 ml), the organic phases were combined and concentrated to give 1.5 g (76.5% yield) of the target product (E)-3-(5-nitro-1-cyclohexen-1-yl)acrylic acid.

900186-90-5
1 suppliers
inquiry

899809-64-4
87 suppliers
inquiry
Yield: 76.5%
Reaction Conditions:
Stage #1:C10H13NO4 with sodium hydroxide in methanol;water at 5 - 20; for 24 h;
Stage #2: with acetic acid in methanol;water; pH=4 - 5
Steps:
2
To a solution of 10 (67 g, 432 mmol) in 1 L of methanol at 0 0C was added 144.4 g (432 mmol) of the Wittig reagent. The resulting mixture was agitated at 0 0C for 3 hrs. The solvent was removed under reduced pressure. The residue was extracted with MeOBu -t twice. The extract was filtered to remove any solid, washed with brine, and concentrated. The residue was chromatographed on a silica gel column, eluting with hexane/ethyl acetate (10/ 1) to give 9.2 g cis and 55.1 g (60.4%) trans product. 1H NMR (CDCl3) d 7.31 (d, J = 11.3 Hz, IH), 6.18 (m, IH), 5.84 (d, J = 15.9 Hz, IH), 4.74-4.68 (m, IH), 3.76 (s, 3H), 2.81-2.74 (m, 2H), 2.50-2.04 (m, 4H).Next, to a flask were added 2.1 g of the methyl ester, 9.6 ml of MeOH and 2.4 ml of water. To the mixture at about 5 0C was added dropwise 0.96 ml of 50% NaOH. The mixture was allowed to warm to room temperature and stirred at this temperature for about 24 hrs. The reaction mixture was neutralized with HOAc to pH between 4 and 5 and the methanol was removed under reduced pressure. The residue was extracted with 3 X 50 ml EtOAc. The EtOAc layer was concentrated to EPO
References:
SCHERING CORPORATION;WU, George, G.;SUDHAKAR, Anantha;WANG, Tao;XIE, Ji;CHEN, Frank, Xing;POIRIER, Marc;HUANG, Mingsheng;SABESAN, Vijay;KWOK, Daw-long;CUI, Jian;YANG, Xiaojing;THIRUVENGADAM, Tiruvettipuram, K.;LIAO, Jing;ZAVIALOV, Ilia;NGUYEN, Hoa, N.;LIM, Ngiap Kie WO2006/76415, 2006, A2 Location in patent:Page/Page column 35-37