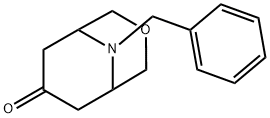
9-Benzyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-one synthesis
- Product Name:9-Benzyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-one
- CAS Number:81514-40-1
- Molecular formula:C14H17NO2
- Molecular Weight:231.29
![3-Oxa-9-azabicyclo[3.3.1]nonan-7-one Hydrochloride](/CAS/GIF/1126795-00-3.gif)
1126795-00-3

100-39-0
![9-Benzyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-one](/CAS/GIF/81514-40-1.gif)
81514-40-1
A solution of potassium carbonate (2.94 g, 14.16 mmol) in DMF (10 mL) was slowly added dropwise at room temperature to a stirred solution of 3-oxa-9-azabicyclo[3.3.1]nonan-7-one hydrochloride (1.26 g, 7.08 mmol, CAS: 1126795-00-3, purchased from PharmaBlock (Nanjing) R&D Co. 14.16 mmol) in DMF (10 mL) solution at room temperature. Benzyl bromide (1.33 g, 7.78 mmol) was then added. The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the mixture was concentrated and the residue was partitioned between water (5 mL) and ethyl acetate (20 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered and concentrated. Purification of the residue by column chromatography afforded the target compound 9-benzyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-one (1.38 g, 84.2% yield). The structure of the product was confirmed by 1H NMR (CDCl3, 400 MHz) and LC/MS: 1H NMR (CDCl3, 400 MHz) δ 7.36 (m, 5H), 3.93 (s, 2H), 3.86 (d, J = 11.0 Hz, 2H), 3.73 (d, J = 10.8 Hz, 2H), 3.18 (d, J = 5.6 Hz, 2H), 2.76 (d, J = 5.6 Hz, 2H), 3.18 (d, J = 5.6 Hz, 2H). 2H), 2.76 (dd, J = 15.9, 5.6 Hz, 2H), 2.35 (d, J = 15.6 Hz, 2H); LC/MS: Calculated value 232 (MH+), Experimental value 232 (MH+).
![3-Oxa-9-azabicyclo[3.3.1]nonan-7-one Hydrochloride](/CAS/GIF/1126795-00-3.gif)
1126795-00-3
53 suppliers
$44.00/100mg

100-39-0
429 suppliers
$10.00/10 g
![9-Benzyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-one](/CAS/GIF/81514-40-1.gif)
81514-40-1
84 suppliers
$60.00/25 mg
Yield: 84.2%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20;
Steps:
10.I Step I
To a stirred solution of 3-oxa-9-azabicyclo[3.3.1]nonan-7-one hydrochloride (1.26 g, 7.08 mmol, cas: 1126795-00-3, purchased from PharmaBlock (Nanjing) R&D Co. Ltd,) and potassium carbonate (2.94 g, 14.16 mmol) in DMF (10 mL) was added benzyl bromide (1.33 g, 7.78 mmol) dropwise at room temperature. The resulting mixture was stirred at room temperature overnight, then concentrated and the residue was partitioned between H20 (5 mL) and EtOAc (20 mL). The organic layer was dried, and then concentrated. The residue was purified to give Compound T (1.38 g, 84.2%). 1H NMR (CDC13, 400 MHz): 7.36 (m, 5H), 3.93 (s, 2H), 3.86 (d, J = 11.0, 2H), 3.73 (d, J = 10.8, 2H), 3.18 (d, J = 5.6 , 2H), 2.76 (dd, J = 15.9, 5.6 Hz, 2H), 2.35 (d, J = 15.6, 2H) ppm. LC/MS: calc'd 232 (MH+), exp 232 (MH+).
References:
F. HOFFMANN-LA ROCHE AG;HOFFMANN-LA ROCHE INC.;GUO, Lei;HU, Taishan;HU, Yimin;KOCER, Buelent;KOU, Buyu;LI, Gangqin;LIN, Xianfeng;LIU, Haixia;SHEN, Hong;SHI, Houguang;WU, Guolong;ZHANG, Zhisen;ZHOU, Mingwei;ZHU, Wei WO2014/184328, 2014, A1 Location in patent:Page/Page column 112
542-05-2
488 suppliers
$6.00/10g

100-46-9
501 suppliers
$5.00/5 g
4358-64-9
104 suppliers
$27.00/1g
![9-Benzyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-one](/CAS/GIF/81514-40-1.gif)
81514-40-1
84 suppliers
$60.00/25 mg

542-05-2
488 suppliers
$6.00/10g

7456-83-9
7 suppliers
inquiry

100-46-9
501 suppliers
$5.00/5 g
![9-Benzyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-one](/CAS/GIF/81514-40-1.gif)
81514-40-1
84 suppliers
$60.00/25 mg

542-05-2
488 suppliers
$6.00/10g
![1,1'-oxybis[2,2-diethoxyethane]](/CAS/GIF/56999-16-7.gif)
56999-16-7
49 suppliers
$90.00/1g

100-46-9
501 suppliers
$5.00/5 g
![9-Benzyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-one](/CAS/GIF/81514-40-1.gif)
81514-40-1
84 suppliers
$60.00/25 mg

542-05-2
488 suppliers
$6.00/10g

1558-67-4
2 suppliers
inquiry

100-46-9
501 suppliers
$5.00/5 g
![9-Benzyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-one](/CAS/GIF/81514-40-1.gif)
81514-40-1
84 suppliers
$60.00/25 mg