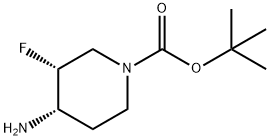
tert-butyl (3R,4S)-4-amino-3-fluoropiperidine-1-carboxylate synthesis
- Product Name:tert-butyl (3R,4S)-4-amino-3-fluoropiperidine-1-carboxylate
- CAS Number:907544-17-6
- Molecular formula:C10H19FN2O2
- Molecular Weight:218.27
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

907544-17-6
64 suppliers
$452.47/5MG
Yield: 99%
Reaction Conditions:
Stage #1:tert-butyl-(3R,4S)-4-(benzylamino)-3-fluoro-piperidine-1-carboxylate with ammonium formate in ethanol;water at 20; for 0.0833333 h;Inert atmosphere;
Stage #2: with palladium 10% on activated carbon in ethanol;water for 1 h;Inert atmosphere;Reflux;
Steps:
29 5.1.28. tert-Butyl (3S,4R)-4-amino-3-fluoropiperidine-1-carboxylate (25)
General procedure: Under N2 gas atmosphere, HCO2NH4 (24.1 g, 382 mmol) andH2O (29.5 mL) were added to a solution of 23 (29.5 g, 95.7 mmol)in EtOH (295 mL), and the mixture was stirred at room temperaturefor 5 min. After adding 10% Pd-C (50% wet) (10.2 g), the mixturewas stirred under reflux for 1 h. After cooling to roomtemperature, the Pd-catalyst was filtrated through a pad ofCelite. The filtrate was concentrated under reduced pressure, andthe residue was purified by column chromatography(CHCl3/MeOH = 100/0 to 90/10) to give the title compound(20.6 g, 99%).
References:
Nakajima, Yutaka;Inoue, Takayuki;Nakai, Kazuo;Mukoyoshi, Koichiro;Hamaguchi, Hisao;Hatanaka, Keiko;Sasaki, Hiroshi;Tanaka, Akira;Takahashi, Fumie;Kunikawa, Shigeki;Usuda, Hiroyuki;Moritomo, Ayako;Higashi, Yasuyuki;Inami, Masamichi;Shirakami, Shohei [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 15,p. 4871 - 4883]
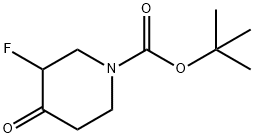
211108-50-8
189 suppliers
$8.00/250mg

907544-17-6
64 suppliers
$452.47/5MG

79099-07-3
0 suppliers
$5.00/5g

907544-17-6
64 suppliers
$452.47/5MG
![tert-Butyl 4-[(Trimethylsilanyl)oxy]-3,6-dihydro-2H-pyridine-1-carboxylate](/CAS/GIF/211108-48-4.gif)
211108-48-4
37 suppliers
$200.00/1g

907544-17-6
64 suppliers
$452.47/5MG