
4-(1-(TERT-BUTOXYCARBONYL)AZETIDIN-3-YL)BENZOIC ACID synthesis
- Product Name:4-(1-(TERT-BUTOXYCARBONYL)AZETIDIN-3-YL)BENZOIC ACID
- CAS Number:908334-10-1
- Molecular formula:C15H19NO4
- Molecular Weight:277.32
![1-AZETIDINECARBOXYLIC ACID, 3-[4-(ETHOXYCARBONYL)PHENYL]-, 1,1-DIMETHYLETHYL ESTER](/CAS/20180601/GIF/439691-97-1.gif)
439691-97-1
3 suppliers
inquiry

908334-10-1
32 suppliers
inquiry
Yield:908334-10-1 86%
Reaction Conditions:
with methanol;sodium hydroxide in tetrahydrofuran at 25 - 60; for 1 h;
Steps:
26.5 4-(1-(tert-Butoxycarbonyl)azetidin-3-yl)benzoic acid
Production Example 26-5
4-(1-(tert-Butoxycarbonyl)azetidin-3-yl)benzoic acid
A 2 M sodium hydroxide solution (28.5 mL, 57.0 mmol) was added to a solution of tert-butyl 3-(4-(ethoxycarbonyl)phenyl)azetidine-1-carboxylate described in Production Example 26-4 (4.35 g, 14.2 mmol) in tetrahydrofuran (32 mL) and methanol (7 mL) at 25° C.
The reaction liquid was stirred at 60° C. for 1 hour. 2 M hydrochloric acid (28.5 mL) was added to the reaction liquid, and the mixture was diluted with ethyl acetate.
The organic layer was washed with a saturated saline solution and then dried over anhydrous sodium sulfate.
The drying agent was separated by filtration and then the filtrate was concentrated under vacuum.
The residue was purified with silica gel column chromatography (n-heptane:ethyl acetate=9:1-1:1) to obtain the title compound (3.4 g, 86%).
1H-NMR Spectrum (CDCl3) δ (ppm): 1.48 (9H, s), 3.71-3.88 (1H, m), 3.96-4.04 (2H, m), 4.37 (2H, t, J=8.6 Hz), 7.42 (2H, d, J=8.4 Hz), 8.09 (2H, d, J=8.3 Hz).
References:
US2014/235614,2014,A1 Location in patent:Paragraph 0579; 0580; 0581
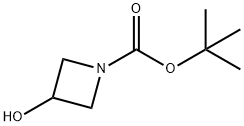
141699-55-0
390 suppliers
$6.00/1g

908334-10-1
32 suppliers
inquiry
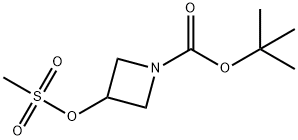
141699-58-3
112 suppliers
$6.00/250mg

908334-10-1
32 suppliers
inquiry
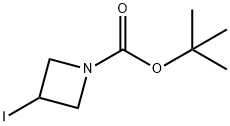
254454-54-1
261 suppliers
$15.00/1g

908334-10-1
32 suppliers
inquiry
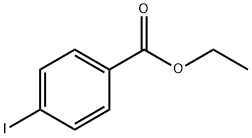
51934-41-9
294 suppliers
$6.00/5g

908334-10-1
32 suppliers
inquiry