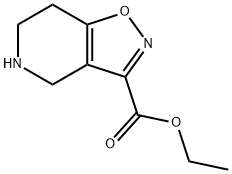
4,5,6,7-TETRAHYDRO-ISOXAZOLO[4,5-C]PYRIDINE-3-CARBOXYLIC ACID, ETHYL ESTER synthesis
- Product Name:4,5,6,7-TETRAHYDRO-ISOXAZOLO[4,5-C]PYRIDINE-3-CARBOXYLIC ACID, ETHYL ESTER
- CAS Number:912330-17-7
- Molecular formula:C9H12N2O3
- Molecular Weight:196.2
![7a-Pyrrolidin-1-yl-3a,6,7,7a-tetrahydro-4H-isoxazolo[4,5-c]pyridine-3,5-dicarboxylic acid 5-tert-butyl ester 3-ethyl ester](/CAS/20180629/GIF/1190971-34-6.gif)
1190971-34-6
2 suppliers
inquiry
![4,5,6,7-TETRAHYDRO-ISOXAZOLO[4,5-C]PYRIDINE-3-CARBOXYLIC ACID, ETHYL ESTER](/CAS/GIF/912330-17-7.gif)
912330-17-7
25 suppliers
inquiry
Yield:912330-17-7 62%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 0;Reflux;
Steps:
1.3 Stage 3: Ethyl 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridin-3-carboxylate
Trifluoroacetic acid (6.67 ml, 89.9 mmol) was added at 0° C. to a solution of 5-tert-butyl 3-ethyl 7a-(pyrrolidin-1-yl)-3a,4,7,7a-tetrahydroisoxazolo[4,5-c]pyridine-3,5(6H)-dicarboxylate (5.5 g, 14.98 mmol) in DCM (100 ml), and the mixture was stirred for 16 hours under reflux. Cooling to 0° C. was then carried out and NaHCO3 solution was added. The phases were separated and the aqueous phase was extracted with dichloromethane. The combined organic phases were dried over Na2SO4, filtered and concentrated in vacuo. Purification of the crude product was carried out by column chromatography (silica gel, 16% methanol in ethyl acetate). Yield: 62% [Alternatively, ethyl 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3-carboxylate A-06 can be synthesised as described in WO2006105945.]
References:
US2010/234340,2010,A1 Location in patent:Page/Page column 23

79099-07-3
0 suppliers
$5.00/5g
![4,5,6,7-TETRAHYDRO-ISOXAZOLO[4,5-C]PYRIDINE-3-CARBOXYLIC ACID, ETHYL ESTER](/CAS/GIF/912330-17-7.gif)
912330-17-7
25 suppliers
inquiry
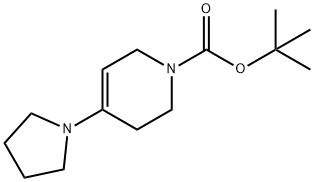
207691-65-4
33 suppliers
$140.68/250mgs:
![4,5,6,7-TETRAHYDRO-ISOXAZOLO[4,5-C]PYRIDINE-3-CARBOXYLIC ACID, ETHYL ESTER](/CAS/GIF/912330-17-7.gif)
912330-17-7
25 suppliers
inquiry