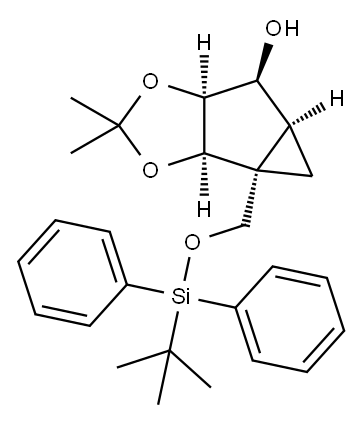
(1R,2R,3S,4S,5S)-1-(tert-Butyldiphenyl)silyloxyMethyl-2,3-dioxy-O,O-isopropylidenebicyclo[3.1.0]hexan-4-ol synthesis
- Product Name:(1R,2R,3S,4S,5S)-1-(tert-Butyldiphenyl)silyloxyMethyl-2,3-dioxy-O,O-isopropylidenebicyclo[3.1.0]hexan-4-ol
- CAS Number:915694-38-1
- Molecular formula:C26H34O4Si
- Molecular Weight:438.63

75-11-6
352 suppliers
$10.00/5g
![(3aS,4S,6aR)-6-((tert-butyldiphenylsilyloxy)methyl)-2,2-dimethyl-4,6a-dihydro-3aH-cyclopenta[d][1,3]dioxol-4-ol](/CAS/20180601/GIF/303963-93-1.gif)
303963-93-1
4 suppliers
inquiry
![(1R,2R,3S,4S,5S)-1-(tert-Butyldiphenyl)silyloxyMethyl-2,3-dioxy-O,O-isopropylidenebicyclo[3.1.0]hexan-4-ol](/CAS/GIF/915694-38-1.gif)
915694-38-1
17 suppliers
$1191.00/1g
Yield:915694-38-1 86%
Reaction Conditions:
Stage #1: (3aS,4S,6aR)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-2,2-dimethyl-4,6a-dihydro-3aH-cyclopenta[d][1,3]dioxol-4-olwith diethylzinc in hexane;dichloromethane at -18; for 0.25 h;Inert atmosphere;
Stage #2: diiodomethane in hexane;dichloromethane at -18;Inert atmosphere;
Steps:
1R,2R,3S,4S,5S)-1-{[(tert-Butyldiphenylsilyl)oxy]methyl-2,3-O-isopropylidenebicyclo [3.1.0]hexan-2,3,4-triol (4).
The procedure was modified according to reference [16]. The (1S,2S,3R)-4-{[(tert-Butyldiphenylsilyl)oxy]methyl}-2,3-O-isopropylidene-4-cyclopenten-1,2,3-triol (1.01 g,2.37 mmol) was dissolved in dry CH2Cl2 (13 mL) under a nitrogen atmosphere. The reactionwas cooled down to 18 C with an ice/salt bath. Diethylzinc (1 mol/L in hexane,2.60 mL, 2.60 mmol, 1.1 eq.) was added dropwise, and the mixture was stirred for 15 min.Diidomethane (0.22 mL, 2.73 mmol, 1.15 eq.) in dry CH2Cl2 (1.6 mL) was also added dropwiseand the reaction was stirred for another 15 min. Both steps were repeated a second time.Then diethylzinc (1 mol/L in hexane, 2.60 mL, 2.60 mmol, 1.1 eq.) was added for the thirdtime. After stirring for 15 min at 18 C, the reaction was allowed to warm to rt and stirredovernight. The reaction was quenched with saturated NH4Cl-solution and was extractedfive times with CH2Cl2. The organic phase was dried over anh. Na2SO4 and concentratedin vacuo. The residue was purified by fc (cyclohexane:ethyl acetate = 7:1, = 5 cm,l = 22 cm, V = 30 mL) to afford the product 4 as a colorless oil (Rf = 0.20, cyclohexane:ethyl acetate = 5:1), yield 0.90 g (86%). C26H34O4Si (438.64 g/mol). Purity (HPLC: methodB): > 99% (tR = 18.94 min). Exact mass (APCI): m/z calculated for C23H27O2Si [M-OH,-CO(CH3)2]+ 363.1775, found 363.1777. 1H-NMR (600 MHz, CDCl3) (ppm) = 7.66-7.60(m, 4H, 2, 6-CHPh), 7.46-7.34 (m, 6H, 3, 4, 5CHPh), 5.00 (dd, J = 6.9, 1.2 Hz, 1H, 2-CH), 4.54(td, J = 6.9, 0.8 Hz, 1H, 3-CH), 4.45 (dt, J = 9.6, 6.1 Hz, 1H, 4CH), 4.12 (q, J = 7.2 Hz, 0.2H,CH2, solvent: ethyl acetate), 4.07 (d, J = 11.0 Hz, 1H, OCHH), 3.29 (d, J = 11.0 Hz, 1H,OCHH), 2.33 (d, J = 9.7 Hz, 1H, OH), 2.04 (s, 0.3H, OCH3, solvent: ethyl acetate), 1.61 (dt,J = 9.3, 4.9 Hz, 1H, 5CH), 1.54 (s, 3H, C(CH3)2), 1.31 (s, 3H, C(CH3)2), 1.26 (t, J = 7.1 Hz,0.5H, CH2CH3, solvent: ethyl acetate), 1.09 (t, J = 5.0 Hz, 1H, 6-CHH), 1.05 (s, 9H, C(CH3)3),0.54 (ddt, J = 8.8, 5.3, 1.1 Hz, 1H, 6-CHH). 13C-NMR (151 MHz, CDCl3) (ppm) = 135.7 (4C,C-2, 6Ph), 133.8, 133.7 (2C, C-1Ph), 129.9 (2C, C-4Ph), 127.8 (4C, C-3, 5Ph), 113.0 (1C, C(CH3)2),81.3 (1C, C2), 79.9 (1C, C3), 71.2 (1C, C4), 65.4 (1C, OCH2), 35.7 (1C, C-1), 33.0 (1C, C-5), 27.0 (3C, C(CH3)3), 26.3 (1C, C(CH3)2), 24.8 (1C, C(CH3)2), 19.4 (1C, C(CH3)3), 10.5 (1C, C-6).FT-IR (neat) v (cm1) = 2932, 2859 (C-Haliphat.), 1470 (C = Caromat.), 1107, 1080, 1042 (CO),741, 702 (CHaromat., out of plane).
References:
Daniliuc, Constantin G.;Heitman, Laura H.;Isaak, Andreas;Jacobson, Kenneth A.;Junker, Anna;Kock, Max;Lemmerhirt, Jan Phillip;Liu, Rongfang [Molecules,2022,vol. 27,# 7,art. no. 2283]
![(3aR,6aR)-6-((tert-butyldiphenylsilyloxy)methyl)-2,2-dimethyl-3aH-cyclopenta[d][1,3]dioxol-4(6aH)-one](/CAS/GIF/303963-92-0.gif)
303963-92-0
17 suppliers
$500.41/5MG
![(1R,2R,3S,4S,5S)-1-(tert-Butyldiphenyl)silyloxyMethyl-2,3-dioxy-O,O-isopropylidenebicyclo[3.1.0]hexan-4-ol](/CAS/GIF/915694-38-1.gif)
915694-38-1
17 suppliers
$1191.00/1g
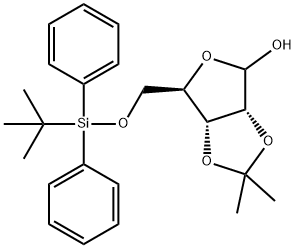
96690-02-7
8 suppliers
inquiry
![(1R,2R,3S,4S,5S)-1-(tert-Butyldiphenyl)silyloxyMethyl-2,3-dioxy-O,O-isopropylidenebicyclo[3.1.0]hexan-4-ol](/CAS/GIF/915694-38-1.gif)
915694-38-1
17 suppliers
$1191.00/1g
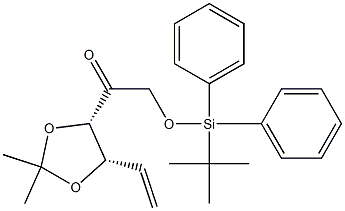
681853-95-2
5 suppliers
inquiry
![(1R,2R,3S,4S,5S)-1-(tert-Butyldiphenyl)silyloxyMethyl-2,3-dioxy-O,O-isopropylidenebicyclo[3.1.0]hexan-4-ol](/CAS/GIF/915694-38-1.gif)
915694-38-1
17 suppliers
$1191.00/1g
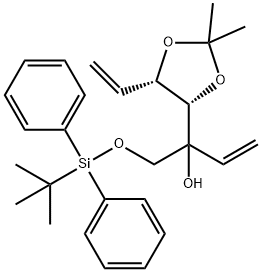
902799-70-6
5 suppliers
inquiry
![(1R,2R,3S,4S,5S)-1-(tert-Butyldiphenyl)silyloxyMethyl-2,3-dioxy-O,O-isopropylidenebicyclo[3.1.0]hexan-4-ol](/CAS/GIF/915694-38-1.gif)
915694-38-1
17 suppliers
$1191.00/1g