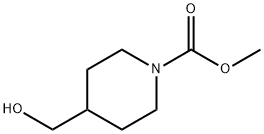
1-Piperidinecarboxylic acid, 4-(hydroxymethyl)-, methyl ester synthesis
- Product Name:1-Piperidinecarboxylic acid, 4-(hydroxymethyl)-, methyl ester
- CAS Number:916078-39-2
- Molecular formula:C8H15NO3
- Molecular Weight:173.21
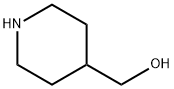
6457-49-4
391 suppliers
$18.00/25g
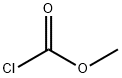
79-22-1
2 suppliers
$38.00/1000g

916078-39-2
25 suppliers
inquiry
Yield:916078-39-2 90%
Reaction Conditions:
with potassium carbonate in water at 0 - 30; for 18 h;Product distribution / selectivity;
Steps:
214.a, 216.a
4-Hydroxymethylpiperidine (1.0 g, 8.6 mmol) was dissolved in water (15 mL) and cooled to 0° C. To this solution was added dropwise a solution of potassium carbonate (4.8 g, 34.7 mmol) in water (10 mL), followed by methyl chloroformate (2.68 mL, 34.7 mmol). The mixture was stirred vigorously and allowed to warm to room temperature over 2 h. After stirring overnight (16 h), the reaction mixture was acidified with 6M aqueous hydrochloric acid and extracted with dichloromethane (3*60 mL). The extracts were combined, dried over sodium sulfate and filtered. The filtrate was evaporated to yield the title intermediate (1.4 g, 8.1 mmol, 93%) as a colorless oil. (m/z): C8H15NO3 calcd. 173.11; found 156.2 [M-H2O+H]+. 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.98 (m, 2H), 1.52 (m, 1H), 1.63 (br d, 2H), 2.72 (br m, 2H), 3.23 (d, 2H), 3.56 (s; 3H), 3.95 (br d, 2H), 4.48 (br s, 1H).; 4-Hydroxymethylpiperidine (47.6 g, 1.0 eq) and water (300 mL) were charged to a flask. The resulting mixture was cooled to 0-10° C. Potassium carbonate (85.7 g, 1.5 eq) dissolved in water (150 mL) and methyl chloroformate (38.4 mL, 1.1 eq) were added while maintaining the temperature at below 10° C. When the addition was complete, the reaction mixture was warmed up to 20-30° C. for 1 hour. After the reaction was complete, dichloromethane (500 mL) was added to the reaction mixture. The organic layer was collected and washed with 1 M phosphoric acid solution (200 mL), saturated sodium bicarbonate solution (200 mL) and saturated sodium chloride solution (200 mL). The organic layer was dried over sodium sulfate (50 g, 1 w/w eq) and then distilled under vacuum to produce the title intermediate. (67.0 g, 90% yield)
References:
US2006/270652,2006,A1 Location in patent:Page/Page column 25-26