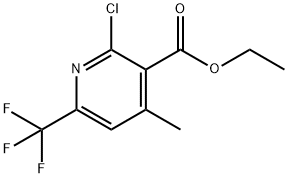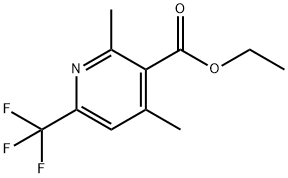
3-Pyridinecarboxylic acid, 2,4-dimethyl-6-(trifluoromethyl)-, ethyl ester synthesis
- Product Name:3-Pyridinecarboxylic acid, 2,4-dimethyl-6-(trifluoromethyl)-, ethyl ester
- CAS Number:916160-53-7
- Molecular formula:C11H12F3NO2
- Molecular Weight:247.21
Yield:916160-53-7 44%
Reaction Conditions:
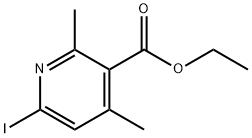
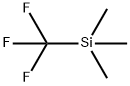
Stage #1: 6-iodo-2,4-dimethyl-nicotinic acid ethyl ester;(trifluoromethyl)trimethylsilanewith potassium fluoride;copper(l) iodide in 1-methyl-pyrrolidin-2-one at 70;
Stage #2: with hydrogen;palladium 10% on activated carbon in ethanol;acetic acid; under 2068.65 Torr; for 48 h;
Steps:
24.3
step 3 - A mixture of Cu(I)I (0.774 g, 4.06 mmol) and previously dried and ground- up KF (0.236 g, 4.06 mmol) was heated with a heat gun under high vacuum until it became an evenly green powder. The powder was cooled to RT before adding 6-iodo- 2,4-dimethyl-nicotinic acid ethyl ester ( 1.26 g, 4.13 mmol, not corrected for impurity vide supra) in NMP ( 10 mL) and TMSCF3 (0.61 ml, 4.12 mmol). The resulting mixture was stirred at 70 C overnight, cooled to RT and poured into a 12% aqueous NH4OH solution. The solution was back extracted with Et2?. The combined organic layers were washed twice with the 12% aqueous NH4OH solution and with brine, dried (Na2SO4), filtered and evaporated. The product could only be isolated from the unreacted halopyridines after hydrogenolysis ( 190 mg 10% Pd/C, HOAc (0.5 mL), EtOH ( 10 mL), 40 psi H2, 48 h). The mixture was filtered and the cake was rinsed with MeOH and the filtrate was evaporated. The residue was purified by SiO2 chromatography eluting with an EtOAc/hexane gradient (0 to 5% EtOAc) to afford 0.405 g (44% yield over two steps) of 82c.
References:
WO2008/34731,2008,A1 Location in patent:Page/Page column 76-77
54453-94-0
22 suppliers
inquiry

916160-53-7
2 suppliers
inquiry
24186-78-5
12 suppliers
$121.00/250mg

916160-53-7
2 suppliers
inquiry