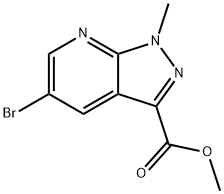
Methyl 5-bromo-1-methyl-1H-pyrazolo[3,4-b]pyridine-3-carboxylate synthesis
- Product Name:Methyl 5-bromo-1-methyl-1H-pyrazolo[3,4-b]pyridine-3-carboxylate
- CAS Number:916326-80-2
- Molecular formula:C9H8BrN3O2
- Molecular Weight:270.08

74-88-4
357 suppliers
$15.00/10g
![Methyl 5-bromo-1-methyl-1H-pyrazolo[3,4-b]pyridine-3-carboxylate](/CAS/GIF/916326-80-2.gif)
916326-80-2
27 suppliers
$297.00/100mg
Yield:916326-80-2 97%
Reaction Conditions:
Stage #1: methyl 5-bromo-1H-pyrazolo[3,4-b]pyridine-3-carboxylatewith sodium hydride in tetrahydrofuran at 0; for 0.25 h;
Stage #2: methyl iodide in tetrahydrofuran at 0 - 20;
Steps:
11
Sodium hydride (47 mg, 60% in mineral oil, 1.18 mmol) was added in portions to a solution of 5-bromo-lΗ-pyrazolo[3,4-b]pyridine-3-carboxylic acid methyl ester Compound Ib (0.3 g, 1.17 mmol) in dry THF (20 mL) at 00C. The mixture was stirred at 0 0C for 15 minutes then methyl iodide (80 μl, 1.29 mmol) was added. The reaction was warmed to room temperature gradually and stirred overnight, the solvent was removed and the residue was purified by silica gel chromatography (10% to 50% of ethyl acetate in hexanes) to yield 5-bromo-l-methyl-lH-pyrazolo[3,4-b]pyridine-3- carboxylic acid methyl ester Compound 11a (0.31 g, 97% yield) as a white powder. 1H NMR (400 MHz, CDCl3) δ 9.89 (s, i), 4.51 (s, 3H), 3.37 (s, 3H); MS (ESI) m/z: 271 (M+H+).
References:
WO2006/130673,2006,A1 Location in patent:Page/Page column 102
![ethyl 5-chloro-[1,2,4]triazolo[4,3-a]pyrimidine-7-carboxylat](/CAS/GIF/916325-84-3.gif)
916325-84-3
64 suppliers
$100.00/100mg

74-88-4
357 suppliers
$15.00/10g
![Methyl 5-bromo-1-methyl-1H-pyrazolo[3,4-b]pyridine-3-carboxylate](/CAS/GIF/916326-80-2.gif)
916326-80-2
27 suppliers
$297.00/100mg
![Methyl 1H-pyrazolo[3,4-b]pyridine-3-carboxylate](/CAS/GIF/916325-83-2.gif)
916325-83-2
68 suppliers
$63.00/100mg
![Methyl 5-bromo-1-methyl-1H-pyrazolo[3,4-b]pyridine-3-carboxylate](/CAS/GIF/916326-80-2.gif)
916326-80-2
27 suppliers
$297.00/100mg