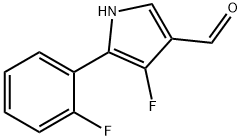
4-fluoro-5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde synthesis
- Product Name:4-fluoro-5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde
- CAS Number:928324-57-6
- Molecular formula:C11H7F2NO
- Molecular Weight:207.18
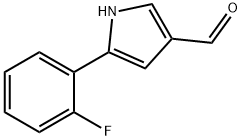
881674-56-2
446 suppliers
$6.00/250mg

928324-57-6
1 suppliers
inquiry
Yield:928324-57-6 13%
Reaction Conditions:
with 1-fluoro-2,6-dichloropyridinium triflate in tetrahydrofuran at 0; for 2 h;
Steps:
115
Reference Example 115 4-fluoro-5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde; To a solution (60 mL) of 5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde (3.1 g) in tetrahydrofuran was added 2,6-dichloro-N-fluoropyridinium triflate (5.6 g) at 0° C., and the mixture was stirred at the same temperature for 2 hr. Saturated aqueous sodium hydrogencarbonate solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The extract was washed with saturated aqueous sodium hydrogencarbonate solution, water and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane-ethyl acetate=2:1) to give the title compound as white crystals (yield 0.43 g, 13%). 1H-NMR (CDCl3) δ: 7.11-7.30 (4H, m), 7.80-7.87 (1H, m), 9.14 (1H, brs), 9.88 (1H, s).
References:
Takeda Pharmaceutical Company Limited US2007/60623, 2007, A1 Location in patent:Page/Page column 38