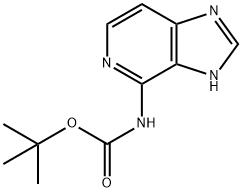
CarbaMic acid, N-3H-iMidazo[4,5-c]pyridin-4-yl-, 1,1-diMethylethyl ester synthesis
- Product Name:CarbaMic acid, N-3H-iMidazo[4,5-c]pyridin-4-yl-, 1,1-diMethylethyl ester
- CAS Number:934816-43-0
- Molecular formula:C11H14N4O2
- Molecular Weight:234.25
![1H-IMidazo[4,5-c]pyridine-1-carboxylic acid, 4-[bis[(1,1-diMethylethoxy)carbonyl]aMino]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/934816-42-9.gif)
934816-42-9
1 suppliers
inquiry
![CarbaMic acid, N-3H-iMidazo[4,5-c]pyridin-4-yl-, 1,1-diMethylethyl ester](/CAS/GIF/934816-43-0.gif)
934816-43-0
19 suppliers
inquiry
Yield:934816-43-0 96%
Reaction Conditions:
Stage #1: 4-(N6,N6-di-tert-butyloxycarbonylamino)-1-tert-butyloxycarbonyl-imidazo[4,5-c]pyridinewith sodium methylate in tetrahydrofuran;methanol at 0; for 0.166667 h;
Stage #2: with hydrogenchloride;water in tetrahydrofuran;methanol; pH=9;
Steps:
2
"Starting material was recovered in 98%. bIt was difficult to separate a desired product from remaining 7The 3 -deaza-tetrazolopurine (19) was converted to the 3-deazaadenine (13) by hydrogenolysis, followed by protection of the amino groups with di-tert-butyl dicarbonate [(BoC)2O] afforded tris-JV-Boc-3-deazaadenine (14) in 94 % yield. Tris-N-Boc-3- deazaadenine (14) was transformed into λ^-Boc-S-deazaadenine (15a) with NaOMe in THF in 96% yield.15a The removal of N9-BoC group of 14 with 1.0 M NaHCO3 in THF gave N6, N6- diBoc-3-deazaadenine (15b) in 92% yield.15bWith protected 3-deazaadenine derivatives (15a and 15b) in hand, Mitsunobu reaction of 5 with 15a or 15b was carefully investigated in the presence of triphenyl phosphme (Ph3P), diisopropyl azodicarboxylate (DIAD) and THF. The coupling reaction of N^-Boc-S- deazaadenine (15a) with 5 provided a mixture of Νg-and Ν7-isomers (16a:17a = 1:4) in 83% yield. In (15b), however, the selectivity toward the Ng- isomer (17b) over the N7-isomer (16b) increased to 10:lin 88% yield, The major product 17b was easily separated from the reaction mixture by silica-gel column chromatography. The removal of Boc, trityl and isopropylidenyl groups of 17b by methanolic HCl gave 3- deazaneplanocin A (2) in 65% overall yield from 6-chloro-3-deazaadenine (14) (7 steps). The regioselectivity of 15b toward the N9-position in the Mitsunobu reaction was enhanced to 5- and 2.5-fold compared with those of 5 and 15a, respectively. Thus, iV^Λ^-diBoc-S-
References:
WO2007/47793,2007,A2 Location in patent:Page/Page column 37-38; 91