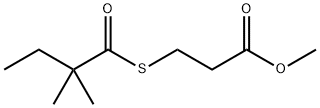
3-[(2,2-Dimethyl-1-oxobutyl)thio]propanoic acid methyl ester synthesis
- Product Name:3-[(2,2-Dimethyl-1-oxobutyl)thio]propanoic acid methyl ester
- CAS Number:938063-63-9
- Molecular formula:C10H18O3S
- Molecular Weight:218.31
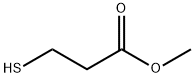
2935-90-2
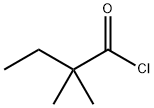
5856-77-9
![3-[(2,2-Dimethyl-1-oxobutyl)thio]propanoic acid methyl ester](/CAS/GIF/938063-63-9.gif)
938063-63-9
To the reaction vessel was added 40 g of 2,2-dimethylbutyryl chloride followed by dropwise addition of 250 ml of dichloromethane and stirred for 15 minutes. After stirring was completed, 15% ice brine was added to the reactor jacket for cooling treatment. When the temperature in the reaction vessel was reduced to 10 °C, 25 g of methyl 3-mercaptopropionate was added and stirring was continued. The temperature was further reduced to induce the reaction of 2,2-dimethylbutyryl chloride with methyl 3-mercaptopropionate to form methyl 3-[(2,2-dimethyl-1-oxobutyl)thio]propionate and hydrogen chloride. When the temperature of the substance in the reaction vessel was reduced to 0°C, ammonia was passed into the reaction vessel until the pH of the reaction system reached 7, and the passage of ammonia was stopped. The reaction mixture was then heated to 65°C and maintained for 1.8 hours to allow the ammonia to react with the hydrogen chloride to form ammonium chloride, thereby promoting completion of the reaction. Upon completion of the reaction, 100 ml of water was added to the reaction vessel and stirred for 30 minutes to ensure that the hydrogen chloride was fully dissolved in water. Afterwards, it was left to stand for 4.5 hours to stratify the liquid. After layering, the aqueous and organic layers were separated and the aqueous layer was an aqueous solution of ammonium chloride. The aqueous layer can be directly transferred or collected to recover the ammonium chloride. Recovery of dichloromethane by distillation gave 55 g of crude product. The crude product was dissolved in 300 ml of ethanol, heated to dissolve and decolorized with activated carbon. The solution was then concentrated and kept at 5°C for 2 hours to give 52 g of the mixture. The mixture was filtered and washed with 78 g of dichloromethane and dried to give a final product of 48.8 g of methyl 3-[(2,2-dimethyl-1-oxobutyl)thio]propionate in 98.4% yield and >99.0% purity.

2935-90-2
333 suppliers
$16.00/5g

5856-77-9
276 suppliers
$34.10/10g
![3-[(2,2-Dimethyl-1-oxobutyl)thio]propanoic acid methyl ester](/CAS/GIF/938063-63-9.gif)
938063-63-9
94 suppliers
$8.00/1g
Yield: 98.4%
Reaction Conditions:
Stage #1:Methyl 3-mercaptopropionate;2,2-dimethylbutanoyl chloride in dichloromethane at 10; for 0.25 h;
Stage #2: with ammonia in dichloromethane; pH=7 at 0 - 65; for 1.8 h;Time;Solvent;
Steps:
1 Example 1
To the reaction vessel was added 40g of 2,2-dimethyl butyryl chloride, and then to the reaction vessel was added dropwise 250ml of dichloromethane, stirred for 15min,After the end of the stirring to the outer jacket of the reactor by adding 15% ice brine cooling cooling treatment, when the temperature in the reaction vessel was cooled to 10 ° C, the reaction vessel was added 25g of 3-mercapto propionic acid, stirring was continued The temperature is lowered so that 2,2-dimethylbutyryl chloride reacts with 3-mercaptopropionate A to produce methyl α-dimethylbutyryl-S-propionate and hydrogen chloride.When the material in the reaction kettle was cooled to 0 ° C, ammonia gas was introduced into the reaction vessel until the pH in the reaction vessel was 7,Ammonia was stopped, heated to 65 ° C and held for 1.8 h,So that ammonia and hydrogen chloride generated by the reaction, ammonium chloride generated to promote the progress of the reaction until the reaction is completed, the reaction vessel was added 100ml of water,After the addition and stirring 30min, making the reaction of hydrogen chloride fully dissolved in water, and then allowed to stand 4.5h, making the dissolved liquid layering.After the liquid is layered, an aqueous layer and an organic layer are obtained, and the aqueous layer is an aqueous solution of ammonium chloride. The aqueous layer of the aqueous layer is directly transferred or collected by an iron drum to recover the ammonium chloride, and the methylene chloride is recovered by distillation to obtain 55 g Of the crude product, and the recovered methylene chloride solution was recycled,55 g of the crude product was dissolved by heating in 300 ml of ethanol, decolored with activated charcoal, concentrated to 5 ° C and held for 2 h to give 52 g of a mixture, 52 g of the mixture was filtered and washed with 78 g of methylene chloride and finally 48.8 g of product was obtained by drying. The yield of methyl α-dimethylbutyryl-S-propionate was 98.4% with a purity of more than 99.0%.
References:
Quzhou Xinbu Chemical Technology Co., Ltd.;Yan Zhaochun CN105837482, 2016, A Location in patent:Paragraph 0031-0034; 0036-0038; 0040-0042; 0044-0046