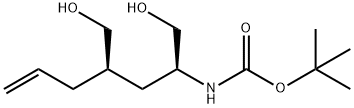
CarbaMic acid, N-[(1S,3R)-1,3-bis(hydroxyMethyl)-5-hexen-1-yl]-, 1,1-diMethylethyl ester synthesis
- Product Name:CarbaMic acid, N-[(1S,3R)-1,3-bis(hydroxyMethyl)-5-hexen-1-yl]-, 1,1-diMethylethyl ester
- CAS Number:942144-12-9
- Molecular formula:C13H25NO4
- Molecular Weight:259.34
153080-81-0
8 suppliers
inquiry
![CarbaMic acid, N-[(1S,3R)-1,3-bis(hydroxyMethyl)-5-hexen-1-yl]-, 1,1-diMethylethyl ester](/CAS/GIF/942144-12-9.gif)
942144-12-9
5 suppliers
inquiry
Yield:942144-12-9 85%
Reaction Conditions:
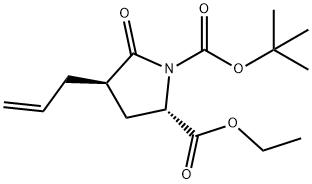
Stage #1: (2S,4R)-1-tert-butyl 4-allyl-2-ethoxycarbonyl-5-oxopyrrolidine-1-carboxylatewith sodium tetrahydridoborate in methanol;lithium hydroxide monohydrate at 20; for 1 h;
Stage #2: with lithium hydroxide monohydrate;ammonia hydrochloride in methanol;
Steps:
1.2
To a solution of (2S,4/?)-l-tert-butyl 2-ethyl 4-allyl-5-oxopyrrolidine-l,2- dicarboxylate (30 g, 0.1 mol) in MeOH/H2O (700/70 mL) was added NaBH4 (25 g, 0.66 mol), the result mixture was stirred 1 hr at rt and quenched with sat. aq. NH4Cl (300 mL). The organic solvent was removed under vacuum and extracted with EtOAc (3*250 mL). The combined organic phases were washed with brine (250 mL) and dried over anhydrous Na2SO4, filtered and evaporated to afford crude tert - butyl (2S,4/?)-l-hydroxy-4-(hydroxymethyl)hept-6-en-2-ylcarbamate (22 g, 85%). It was used in the next step without further purification.
References:
WO2008/156817,2008,A2 Location in patent:Page/Page column 88-89