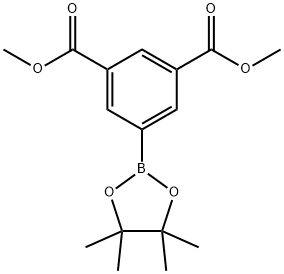
3,5-Bis(methoxycarbonyl)phenylboronic acid pinacol ester synthesis
- Product Name:3,5-Bis(methoxycarbonyl)phenylboronic acid pinacol ester
- CAS Number:944392-68-1
- Molecular formula:C16H21BO6
- Molecular Weight:320.15
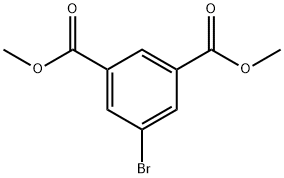
51760-21-5

73183-34-3

944392-68-1
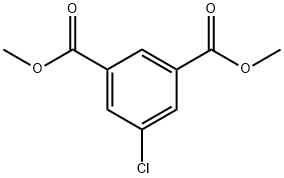
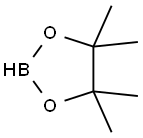
General procedure for the synthesis of 3,5-dimethoxycarbonylphenylboronic acid pinacol ester from dimethyl 5-bromoisophthalate and pinacol ester of bisboronic acid: dimethyl 5-bromo-1,3-benzenedicarboxylate (5.4 g, 19.8 mmol) and pinacol ester of boronic acid (6.0 g, 29.4 mmol) were added into a 250 mL three-necked flask and anhydrous 1,4-dioxane (50 mL) was added As a solvent, Pd(dppf)2Cl2 (0.2 g, 0.27 mmol) was added under nitrogen protection. The reaction mixture was heated to 100 °C and kept for 12 hours. Upon completion of the reaction, it was cooled to room temperature and the excess 1,4-dioxane was removed by distillation. Water (20 mL) was added and extracted with ethyl acetate (30 mL x 3), the organic phases were combined and dried with anhydrous Na2SO4. After filtration, the filtrate was evaporated to obtain the crude product. The crude product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate=94/6) to afford 3,5-dimethoxycarbonylphenylboronic acid pinacol ester (4.59 g, 72% yield) in white powder form.
1459-93-4
346 suppliers
$11.00/100g

73183-34-3
586 suppliers
$6.00/5g

944392-68-1
89 suppliers
$18.00/100mg
Yield: 99%
Reaction Conditions:
with bis(1,5-cyclooctadiene)diiridium(I) dichloride;1-(4,4'-di-tert-butyl-[2,2'-bipyridin]-6-yl)-2-(dimethyl(phenyl)silyl)-2,3-dihydro-1H-benzo[d][1,3,2]diazaboroleInert atmosphere;Schlenk technique;Sealed tube;
Steps:
4.4. General Procedure for Iridium-Catalyzed arene C-H borylation Using NNB-type Ligand
General procedure: B2pin2, [IrCl(COD)]2 (1.0 mol%), preligand 1 (2.0 mol%), and (hetero)arene (0.2 mmol, ifsolid) were placed in a dried Schlenk flask (15 mL in volume) equipped with a stirring bar. Afterevacuating and refilling the flask with dry nitrogen three times, (hetero)arene (0.2 mmol, if liquid)and methoxycyclopentane (CAPE, 0.5 mL) were added with syringes under a stream of nitrogen.The resulting mixture was stirred at the corresponding temperature for the assigned time. After cooling to room temperature, the reaction mixture was concentrated and then purified by columnchromatography on silica gel to give the target product (The spectra data for the borylated compoundscan be seen in the Supplementary Material).
References:
Ding, Siyi;Wang, Linghua;Miao, Zongcheng;Li, Pengfei [Molecules,2019,vol. 24,# 7,art. no. 1434]

51760-21-5
191 suppliers
$6.00/1g

73183-34-3
586 suppliers
$6.00/5g

944392-68-1
89 suppliers
$18.00/100mg
51839-15-7
135 suppliers
$6.00/250mg

73183-34-3
586 suppliers
$6.00/5g

944392-68-1
89 suppliers
$18.00/100mg
20330-90-9
89 suppliers
$45.00/50mg

73183-34-3
586 suppliers
$6.00/5g

944392-68-1
89 suppliers
$18.00/100mg

51839-15-7
135 suppliers
$6.00/250mg
25015-63-8
216 suppliers
$22.39/5G

944392-68-1
89 suppliers
$18.00/100mg