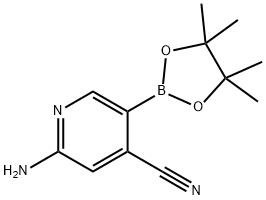
2-aMino-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)isonicotinonitrile synthesis
- Product Name:2-aMino-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)isonicotinonitrile
- CAS Number:944401-73-4
- Molecular formula:C12H16BN3O2
- Molecular Weight:245.09
Yield:944401-73-4 100%
Reaction Conditions:
with potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in 1,4-dioxane at 120; for 2 h;Sealed vessel;
Steps:
11
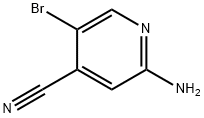
0279] In a glass pressure vessel, a mixture of 2-amino-5-bromo-isonicotinonitrile(25 mg, 0.126 mmol), 4,4>5,5-tetramethyl-2-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2- yl)-l,3,2-dioxaborolane (38 mg, 0.151 mmol) and potassium acetate (49 mg, 0.504 mmol) were suspended in dioxane (1.8 mL). After purging the mixture with argon for 1-2 min, dichloro[l,r-bis(diphenylρhosphino)ferrocene] palladium(II) dichloromethane adduct (16 mg, 0.019 mmol) was added in one portion. The reaction vessel was sealed and heated at 1200C with stirring for 2 hours. The crude reaction solution was cooled to room temperature and used without further purification assuming a quantitative yield of the boronic ester (0.126 mmol). LC/MS (m/z): 164.0 (MH+ of the boronic acid formed by product hydrolysis on LC), Rt 0.37 minutes.
References:
WO2007/84786,2007,A1 Location in patent:Page/Page column 104