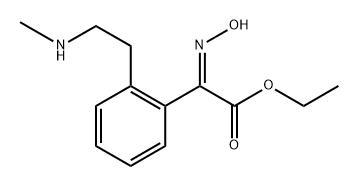
Benzeneacetic acid, α-(hydroxyiMino)-2-[2-(MethylaMino)ethyl]-, ethyl ester synthesis
- Product Name:Benzeneacetic acid, α-(hydroxyiMino)-2-[2-(MethylaMino)ethyl]-, ethyl ester
- CAS Number:945652-12-0
- Molecular formula:C13H18N2O3
- Molecular Weight:250.3
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Benzeneacetic acid, α-(hydroxyiMino)-2-[2-(MethylaMino)ethyl]-, ethyl ester](/CAS/20211123/GIF/945652-12-0.gif)
945652-12-0
2 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:1-ethoxycarbonyl-2-methyl-3,4-dihydroisoquinolinium monomethylsulfate with hydroxylamine hydrochloride in water at 20; for 6 h;
Stage #2: with sodium hydroxide;water in ethyl acetate; pH=9 - 10 at 5; for 0.5 h;Product distribution / selectivity;
Steps:
A2
[Example A2] Preparation of ethyl hydroxyimino-[2-(2-methylaminoethyl)phenyl]acetate The 1-ethoxycarbonyl-3,4-dihydro-2-methylisoquinolinium monomethylsulfate solution obtained by Reference Example A4 was added with 60 ml of water and 22.4 g (322 mmol) of hydroxylamine hydrochloride in a flask. The mixture was stirred at room temperature for six hours. The reaction mixture was added with 120 ml of ethyl acetate, and then the pH was adjusted at 9 to 10 with about 103 g of aqueous sodium hydroxide solution (25%) to yield precipitation. The solution was stirred at 5°C for 30 minutes. Then, the precipitation was filtered, washed with ethyl acetate, and dried to obtain 42.2 g (169 mmol, yield 55%, 3 steps) of ethyl hydroxyimino-[2-(2-methylaminoethyl)phenyl]acetate as white crystals.
References:
API Corporation EP1985615, 2008, A1 Location in patent:Page/Page column 17
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Benzeneacetic acid, α-(hydroxyiMino)-2-[2-(MethylaMino)ethyl]-, ethyl ester](/CAS/20211123/GIF/945652-12-0.gif)
945652-12-0
2 suppliers
inquiry