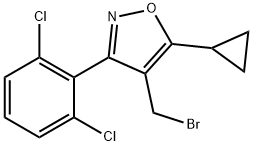
4-BROMOMETHYL-5-CYCLOPROPYL-3-(2,6-DICHLORO-PHENYL)-ISOXAZOLE(WXG03484) synthesis
- Product Name:4-BROMOMETHYL-5-CYCLOPROPYL-3-(2,6-DICHLORO-PHENYL)-ISOXAZOLE(WXG03484)
- CAS Number:946426-94-4
- Molecular formula:C13H10BrCl2NO
- Molecular Weight:347.03
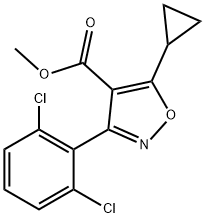
946426-88-6
22 suppliers
$498.49/5MG

946426-94-4
9 suppliers
inquiry
Yield:946426-94-4 96%
Reaction Conditions:
Stage #1: methyl 5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazole-4-carboxylatewith diisobutylaluminium hydride in tetrahydrofuran;toluene at 0 - 20; for 6 h;
Stage #2: with carbon tetrabromide;triphenylphosphine in dichloromethane at 20; for 4 h;Inert atmosphere;
Steps:
IV-1 (21.6 g, 69 mmol) was dissolved in tetrahydrofuran (140 mL), cooled to 0 °C, and a solution of diisobutylaluminum hydride in toluene (1.5 M, 102 mL) was slowly added dropwise to the solution, and the reaction solution was at room temperature. Stir for 6h. The reaction solution was slowly poured into ice water, and 1M aqueous hydrochloric acid was added to adjust the pH to be approximately equal to 2, extracted with ethyl acetate (100 mL each, three times in total), concentrated, and the intermediate alcohol was obtained by column chromatography; This intermediate and triphenylphosphine (59 mmol) were dissolved in dichloromethane (60 mL), cooled to 0°C, and a solution of carbon tetrabromide (62 mmol) in dichloromethane (60 mL) was added dropwise under nitrogen protection , and reacted at room temperature for 4h. The solvent was removed from the reaction solution to obtain an oily substance, which was subjected to column chromatography to obtain Intermediate V-1 (15.3 g, yield 96%).
References:
CN114195777,2022,A Location in patent:Paragraph 0151-0152; 0155
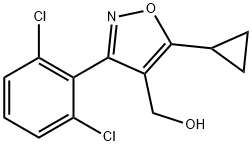
946426-89-7
59 suppliers
inquiry

946426-94-4
9 suppliers
inquiry
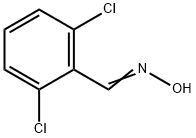
25185-95-9
131 suppliers
$30.80/5g

946426-94-4
9 suppliers
inquiry
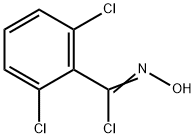
6579-27-7
67 suppliers
$45.00/100mg

946426-94-4
9 suppliers
inquiry
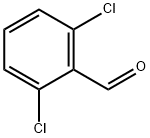
83-38-5
456 suppliers
$6.00/10g

946426-94-4
9 suppliers
inquiry