
4-CHLORO-3-NITRO-6-(TRIFLUOROMETHYL)PYRIDIN-2(1H)-ONE synthesis
- Product Name:4-CHLORO-3-NITRO-6-(TRIFLUOROMETHYL)PYRIDIN-2(1H)-ONE
- CAS Number:947144-53-8
- Molecular formula:C6H2ClF3N2O3
- Molecular Weight:242.54
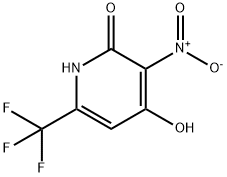
947144-26-5
33 suppliers
inquiry

947144-53-8
4 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 6-trifluoromethyl-3-nitro-2,4-dihydroxypyridinewith P,P-dichlorophenylphosphine oxide at 100; for 19 h;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate at 0; pH=~ 8;
Steps:
4
3-Nitro-6-trifluoromethyl-pyridine-2,4-diol (5.8 gm, 26 mmol) was heated in phenylphosphonic dichloride (3OmL) at 1000C for 19 hours. Cooled and poured on to ice (60 gm), extracted with ethyl acetate (3 x 50 mL). The combined organic extracts were washed with aqueous sodium hydrogen carbonate solution (10%w/v) until the washings remained basic (pH ~8). The deep yellow organic layer was then washed with saturated brine, dried over sodium sulphate, filtered and evaporated to give a yellow gum. Trituration of the gum with dichloromethane gave a yellow solid which was filtered off and dried (4.65gm). The solid was dissolved in water (25mL) and acidified with 2N hydrochloric acid (7.5mL) to give a thick white precipitate which was filtered off and washed with water. The precipitate was dissolved in ethyl acetate, dried over sodium sulphate, filtered and evaporated to give 4-chloro-3-nitro-6-trifluoromethyl- pyridin-2-as a white solid (3.75gm).
References:
WO2008/47201,2008,A2 Location in patent:Page/Page column 32