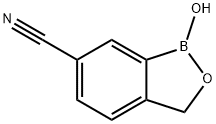
1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole-6-carbonitrile synthesis
- Product Name:1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole-6-carbonitrile
- CAS Number:947162-60-9
- Molecular formula:C8H6BNO2
- Molecular Weight:158.95
![Benzonitrile, 4-[(acetyloxy)methyl]-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-](/CAS/20210305/GIF/1268336-25-9.gif)
1268336-25-9
0 suppliers
inquiry
![1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole-6-carbonitrile](/CAS/20180703/GIF/947162-60-9.gif)
947162-60-9
19 suppliers
inquiry
Yield:947162-60-9 85%
Reaction Conditions:
Stage #1: 4-cyano-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl acetatewith sodium hydroxide in methanol at 30; for 2 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water; for 0.166667 h;
Steps:
41
A solution of sodium hydroxide (13.1 g, 327 mmol) in methanol (300 mL) was added dropwise to a solution of 4-cyano-2-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-ly)benzyl acetate (44.8 g, 149 mmol) in methanol (300 mL) at 30 C. The reaction mixture was stirred for additional 2 hours. The solvent was evaporated and the residue was dissolved in tetrahydrofuran (200 mL). 2 M Aqueous hydrochloric acid (660 mL) was added and the resulting suspension was stirred for 10 minutes. The suspension was cooled down to 10 °C and filtered. The filter cake was washed by water (100 mL) and n-hexane (100 mL) to give 1- hydroxy-1 ,3-dihydrobenzo[c][1 ,2]oxaborole-6-carbonitrile as white powder. Yield: 20.15 g (85%). 1H NMR spectrum (300 MHz, DMSO-d6, 5H): 9.55 (bs, 1 H); 8.09 (s, 1 H); 7.90 (d, J-8.1 Hz, 1 H); 7.63 (d, J=7.9 Hz, 1 H); 5.07 (s, 2 H).
References:
WO2020/201041,2020,A2 Location in patent:Page/Page column 161
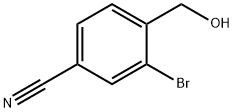
90110-98-8
82 suppliers
inquiry
![1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole-6-carbonitrile](/CAS/20180703/GIF/947162-60-9.gif)
947162-60-9
19 suppliers
inquiry
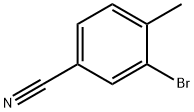
42872-74-2
192 suppliers
$9.00/5g
![1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole-6-carbonitrile](/CAS/20180703/GIF/947162-60-9.gif)
947162-60-9
19 suppliers
inquiry
![Benzonitrile, 4-[(acetyloxy)methyl]-3-bromo-](/CAS/20210305/GIF/201342-56-5.gif)
201342-56-5
0 suppliers
inquiry
![1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole-6-carbonitrile](/CAS/20180703/GIF/947162-60-9.gif)
947162-60-9
19 suppliers
inquiry
