
4-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine synthesis
- Product Name:4-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine
- CAS Number:950511-16-7
- Molecular formula:C22H24B2N2O2
- Molecular Weight:370.07
![2-(4-Chlorophenyl)-2,3-dihydro-1H-naphtho-[1,8-de][1,3,2]diazaborinine](/CAS/GIF/1336918-65-0.gif)
1336918-65-0
14 suppliers
$45.00/50mg

73183-34-3
587 suppliers
$6.00/5g
![4-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine](/CAS/GIF/950511-16-7.gif)
950511-16-7
17 suppliers
$39.00/250mg
Yield:950511-16-7 82%
Reaction Conditions:
with (2,2,2-trifluoroethoxy)trimethylsilane;cesium fluoride;dichlorobis(trimethylphosphine)nickel in tetrahydrofuran at 100; for 12 h;Inert atmosphere;Sealed tube;
Steps:
35 Example-35
Under an argon atmosphere,4.2 mg (0.015 mmol) of dichlorobis (trimethylphosphine) nickel,139 mg (0.5 mmol) of 2- (4-chlorophenyl) -2,3-dihydro-1 H-naphtho [1,8- de] -1,3,2-diazoborinine,152 mg (1.0 mmol) of cesium fluoride,140 mg (0.55 mmol) of 4,4,5,5,4 ', 4', 5 ', 5'-octamethyl-2,2'-bi (1,3,2-dioxaborolanyl)180 mg (1.05 mmol) of trimethyl (2,2,2-trifluoroethoxy) silane and 0.5 mL of tetrahydrofuran were added and sealed,And the mixture was stirred at 100 ° C. for 12 hours.After the reaction vessel was cooled to room temperature, 1 mL of a saturated aqueous solution of ammonium chloride was added, and the mixture was extracted three times with 8 mL of ethyl acetate, and the obtained organic phases were combined. The solvent was distilled off under reduced pressure, and the residue was purified using silica gel column chromatography (hexane: chloroform: ethyl acetate = 4: 1: 0 to 4: 1: 1)2- [4- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) phenyl] -2,3- dihydro-1 H-naphtho [1,8-de] -1 , 3,2-diazoborinine153 mg (white solid, yield 82%) was obtained.
References:
JP5699037,2015,B2 Location in patent:Paragraph 0113; 0114
![2-(4-Bromophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborine](/CAS/GIF/927384-44-9.gif)
927384-44-9
54 suppliers
$20.00/100mg

73183-34-3
587 suppliers
$6.00/5g
![4-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine](/CAS/GIF/950511-16-7.gif)
950511-16-7
17 suppliers
$39.00/250mg
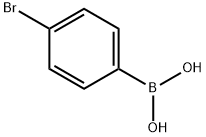
5467-74-3
468 suppliers
$8.00/1g
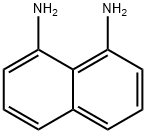
479-27-6
376 suppliers
$6.00/10g
![4-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine](/CAS/GIF/950511-16-7.gif)
950511-16-7
17 suppliers
$39.00/250mg