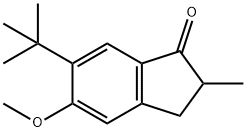
6-(tert-Butyl)-5-methoxy-2-methyl-2,3-dihydro-1H-inden-1-one synthesis
- Product Name:6-(tert-Butyl)-5-methoxy-2-methyl-2,3-dihydro-1H-inden-1-one
- CAS Number:952516-22-2
- Molecular formula:C15H20O2
- Molecular Weight:232.32
Yield:952516-22-2 65%
Reaction Conditions:
with Eaton′s Reagent at 50 - 55; for 2 h;Cooling with ice;Time;Concentration;
Steps:
6-tert-Butyl-5-methoxy-2-methylindan-l-one (second experiment)
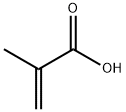
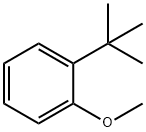
6-terf-Butyl-5-methoxy-2-methylindan-l-one (second experiment) To Eaton's reagent obtained from 118 g of P4O10 and 600 ml of methanesulfonic acid a mixture of 70.3 g (0.428 mol) of l-tert-butyl-2-methoxybenzene and 295.0 g (3.43 mol, 8 eqv.) of methacrylic acid was added for ca. 1 h at 50-55°C. The resulting mixture was stirred for 0.5 h at this temperature, then cooled to room temperature, and poured on a mixture of 1.5 liter of cold water and 2 kg of ice. After the ice melts, the precipitated crude 6-tert-butyl-5-methoxy-2-methylindan-l-one was filtered off and then washed with 2x100 ml of cold water. The crude product was dissolved in 500 ml of dichloromethane, and this solution was washed by aqueous K2CO3, dried over anhydrous K2CO3, and then evaporated on Rotavap. The residue was distilled in vacuum to give 70.6 g of crude 6-tert-butyl-5-methoxy-2- methylindan-l-one, b.p. 155-165°C/5 mm Hg. This product was dissolved in 200 ml of hot hexanes. Crystals precipitated from this solution at 5°C were collected, washed by 50 ml of cold hexanes, and dried in vacuum. This procedure gave 64.1 g (65%) of the analytically pure substituted indanone.
References:
WO2013/7650,2013,A1 Location in patent:Page/Page column 45; 46