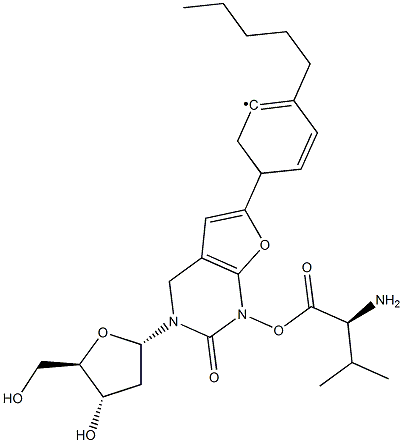
L-Valine 5'-ester with 3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-(4-pentylphenyl)furo[2,3-d]pyrimidin-2(3H)-one synthesis
- Product Name:L-Valine 5'-ester with 3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-(4-pentylphenyl)furo[2,3-d]pyrimidin-2(3H)-one
- CAS Number:956483-02-6
- Molecular formula:C27H35N3O6
- Molecular Weight:497.58
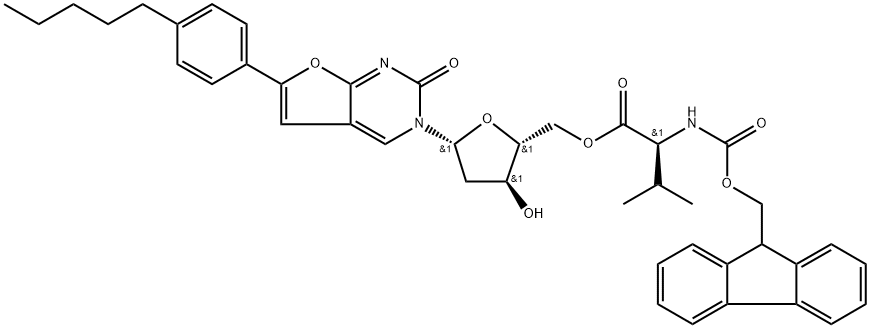
1269409-87-1
7 suppliers
inquiry
![L-Valine 5'-ester with 3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-(4-pentylphenyl)furo[2,3-d]pyrimidin-2(3H)-one](/CAS/20150408/GIF/956483-02-6.gif)
956483-02-6
13 suppliers
inquiry
Yield:956483-02-6 90%
Reaction Conditions:
Stage #1: 3-[2'-deoxy-5'-O-[N-(fluorenylmethoxycarbonyl)valyl]-β-D-ribofuranosyl]-6-(p-pentylphenyl)-2,3-dihydrofuro[2,3-d]pyrimidin-2-onewith 1,8-diazabicyclo[5.4.0]undec-7-ene in dichloromethane at 20; for 2 h;
Stage #2: with hydrogenchloride in dichloromethane;isopropyl alcohol at 5; pH=1 - 4;
Steps:
19
[00225] Example 19Preparation of ( )-((2R,3 ,5R)-(3-Hvdroxy-5-(2-oxo-6-(4-pentylphenyl)furor2,3- rf1pyrimidin-3(2H)-yl)-tetrahvdrofuran-2-yl)methyl 2-amino-3-methylbutanoate[00226] To a 500 mL flask was added DCM 200 mL, (S)-((2R,3S,5R)-3-hydroxy) 5-(2- oxo-6-(4-pentylphenyl)furo[2,3-(i]pyrimidin-3(2H)-yl)-tetrahydrofuran-2- yl)methyl 2-(((9H-fluoren-9-yl)methoxy)carbonylamino)-3-methylbutanoate 19.7 g (27.4 mmol), The reaction mixture was cooled, and 8.3 g DBU (54.8 mmol) was added to the reaction mixture. The reaction was stirred at rt for 2 h until TLC showed the end of the reaction.[00227] TLC: eluant: DCM/methanol=6: 1 ;(lS,)-((2R,35,,5R)-3-hydroxy-5-(2-oxo-6-(4-pentylphenyl)furo[2,3-(i]pyrimidin- 3(2H)-yl)-tetrahydrofuran-2-yl)methyl2-(((9H-fluoren-9-yl)methoxy) carbonylamino)-3-methyl butanoate:R =0.55; (S)-((2R,3S,5R)- (3-hydroxy-5-(2-oxo- 6-(4-pentylphenyl)furo [2,3-J]pyrimidin-3(2H)-yl)-tetrahydrofuran-2-yl))methyl 2-amino- 3-methylbutanoate:R/=0.24.[00228] The above reaction mixture was cooled below 5 °C and was added with 20% ofHQ IPA solution until pH 1-4. The mixture was stirred for additional 1 h and was filtered. The filter cake was washed with dichloromethane. Desired crude product was obtained. The yield was 90% and the purity was 97%. The crude product was recrystallized with methanol/dichloromethane/MTBE to give the desired pure product.
References:
WO2012/48202,2012,A2 Location in patent:Page/Page column 51-52

1370288-89-3
1 suppliers
inquiry
![L-Valine 5'-ester with 3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-(4-pentylphenyl)furo[2,3-d]pyrimidin-2(3H)-one](/CAS/20150408/GIF/956483-02-6.gif)
956483-02-6
13 suppliers
inquiry
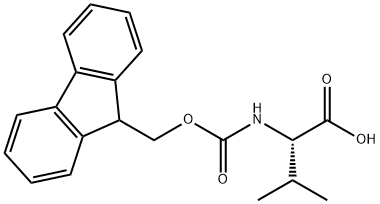
68858-20-8
541 suppliers
$8.00/1g
![L-Valine 5'-ester with 3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-(4-pentylphenyl)furo[2,3-d]pyrimidin-2(3H)-one](/CAS/20150408/GIF/956483-02-6.gif)
956483-02-6
13 suppliers
inquiry