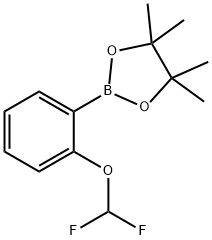
2-(2-(Difluoromethoxy)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane synthesis
- Product Name:2-(2-(Difluoromethoxy)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
- CAS Number:960067-33-8
- Molecular formula:C13H17BF2O3
- Molecular Weight:270.08
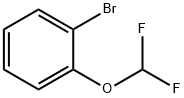
175278-33-8

73183-34-3

960067-33-8
The general procedure for the synthesis of 2-difluoromethoxyphenylboronic acid pinacol ester from o-bromodifluoromethoxybenzene and bis(pinacolato)diboron (1.71 g, 6.75 mmol) was carried out as follows: o-bromodifluoromethoxybenzene (1 g, 4.5 mmol) and bis(pinacolato)diboron (1.71 g, 6.75 mmol) were dissolved in dioxane (10 mL), and followed by addition of anhydrous potassium acetate (1.1 g, 13.53 mmol ) and [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex (369 mg, 0.45 mmol). The reaction mixture was heated to 85 °C under nitrogen protection and stirred continuously for 16 hours. Upon completion of the reaction, the mixture was cooled to room temperature and subsequently concentrated under reduced pressure. The residue was purified by silica gel column chromatography with an eluent ratio of petroleum ether: ethyl acetate = 10:1, resulting in the light yellow oily product 2-difluoromethoxyphenylboronic acid pinacol ester (1.1 g, 90.2% yield).

175278-33-8
143 suppliers
$17.00/5g

73183-34-3
587 suppliers
$6.00/5g

960067-33-8
60 suppliers
$51.00/100mg
Yield: 90.2%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 85; for 16 h;Inert atmosphere;
Steps:
10 Synthesis of Compound 10-a
2-Bromodifluoromethylphenylether (1 g, 4.5 mmol) and bis(pinacolato)diboron (1.71 g, 6.75 mmol) were dissolved in dioxane (10 mL), and anhydrous potassium acetate (1.1 g, 13.53 mmol) and [1,1'-bis (diphenylphosphino)ferrocene]dichloropalladium.dichloromethane (369 mg, 0.45 mmol) were added.
Under nitrogen gas atmosphere, the mixture was heated to 85° C. and stirred for 16 hours.
After cooling to room temperature, the reaction was concentrated under reduced pressure.
The residue was purified by silica gel column chromatography (petroleum ether: ethyl acetate=10:1) to give 10-a as a pale yellow oil (1.1 g, yield 90.2%).
References:
GUANGZHOU MAXINOVEL PHARMACEUTICALS CO., LTD.;XU, Zusheng;ZHANG, Nong;WANG, Tinghan;SUN, Qingrui;WANG, Yuguang US2018/208604, 2018, A1 Location in patent:Paragraph 0216-0217

175278-33-8
143 suppliers
$17.00/5g
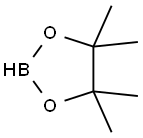
25015-63-8
216 suppliers
$22.39/5G

960067-33-8
60 suppliers
$51.00/100mg
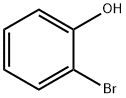
95-56-7
307 suppliers
$9.00/5 g

960067-33-8
60 suppliers
$51.00/100mg