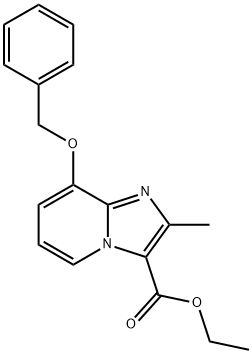
8-BENZYLOXY-2-METHYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:8-BENZYLOXY-2-METHYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:96428-50-1
- Molecular formula:C18H18N2O3
- Molecular Weight:310.35
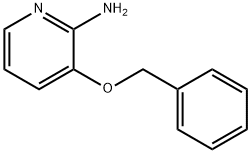
24016-03-3
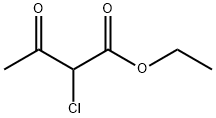
609-15-4
![8-BENZYLOXY-2-METHYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/96428-50-1.gif)
96428-50-1
Example 1A Synthesis of ethyl 8-(benzyloxy)-2-methylimidazo[1,2-a]pyridine-3-carboxylate: 25 g (124.8 mmol) of 2-amino-3-benzyloxypyridine was dissolved in 781 ml of anhydrous ethanol, followed by the addition of 102.7 g (624.2 mmol) of ethyl 2-chloroacetoacetate and 2 tablespoons of 4A molecular sieves. The reaction mixture was heated to reflux (oil bath temperature 100 °C) for 2 days. Upon completion of the reaction, the mixture was concentrated and excess ethyl 2-chloroacetoacetate was removed by rotary evaporator under dry ice cooling conditions. The residue was purified by silica gel column chromatography with a mobile phase of cyclohexane: ethyl acetate (gradient elution from 9:1 to 4:1). Finally, 20.81 g of the target compound ethyl 2-methyl-8-(benzyloxy)imidazo[1,2-a]pyridine-3-carboxylate was obtained in 54% yield and 99% purity. The product was analyzed by LC-MS (Method 2): retention time Rt = 1.12 min, mass spectrum (ESpos): m/z = 311 (M + H)+. 1H NMR (400 MHz, DMSO-d6) data were as follows: δ= 1.35 (t, 3H), 2.59 (s, 3H), 4.34 (q, 2H), 5.32 (s, 2H). 7.01-7.09 (m, 2H), 7.33-7.48 (m, 3H), 7.52 (d, 2H), 8.81-8.86 (m, 1H).

24016-03-3
358 suppliers
$5.00/1g

609-15-4
458 suppliers
$6.00/25g
![8-BENZYLOXY-2-METHYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/96428-50-1.gif)
96428-50-1
49 suppliers
$49.00/100mg
Yield:96428-50-1 54%
Reaction Conditions:
in ethanol at 100; for 48 h;Molecular sieve;
Steps:
1A Ethyl 8-(benzyloxy)-2-methylimidazo[1,2-a]pyridine-3-carboxylate
Example 1A
Ethyl 8-(benzyloxy)-2-methylimidazo[1,2-a]pyridine-3-carboxylate
25 g (124.8 mmol) of 2-amino-3-benzyloxypyridine were dissolved in 781 ml of ethanol, 102.7 g (624.2 mmol) of ethyl 2-chloroacetoacetate and two tablespoons of 4 A molecular sieve were added, and the reaction mixture was then heated at reflux (bath temperature 100° C.) for 2 days.
The mixture was concentrated, and excess ethyl 2-chloroacetoacetate was removed on a rotary evaporator with dry ice cooling.
The residue was purified by silica gel chromatography (mobile phase cyclohexane:ethyl acetate gradient 9:1, 4:1).
This gave 20.81 g of the target compound (54% of theory, purity 99%).
LC-MS (Method 2): Rt=1.12 min
MS (ESpos): m/z=311 (M+H)+
1H NMR (400 MHz, DMSO-d6): δ=1.35 (t, 3H), 2.59 (s, 3H), 4.34 (q, 2H), 5.32 (s, 2H), 7.01-7.09 (m, 2H), 7.33-7.48 (m, 3H), 7.52 (d, 2H), 8.81-8.86 (m, 1H).
References:
US2014/128386,2014,A1 Location in patent:Paragraph 0984; 0985; 0986; 0987; 0988
537-46-2
0 suppliers
$29.50/1mL

24016-03-3
358 suppliers
$5.00/1g

609-15-4
458 suppliers
$6.00/25g
![8-BENZYLOXY-2-METHYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/96428-50-1.gif)
96428-50-1
49 suppliers
$49.00/100mg