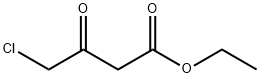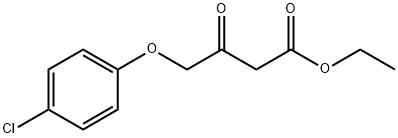
4-(4-CHLOROPHENOXY)-3-OXO-BUTANOIC ACID ETHYL ESTER synthesis
- Product Name:4-(4-CHLOROPHENOXY)-3-OXO-BUTANOIC ACID ETHYL ESTER
- CAS Number:97900-77-1
- Molecular formula:C12H13ClO4
- Molecular Weight:256.68
Yield:97900-77-1 25%
Reaction Conditions:
Stage #1: ethyl acetatewith n-butyllithium;diisopropylamine in hexane at 0; for 1 h;
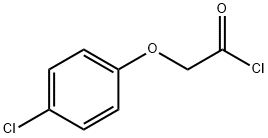
Stage #2: 4-chlorophenyloxyacetyl chloride in tetrahydrofuran;hexane at -78 - 20;
Steps:
4.9.a
EtOAc (0.63 mL. 6.44 mmol) was added to a solution of n-BuLi (1.6 M in hexanes, 9.2 mL, 14.72 mmol) and diisopropylamine (2.1 mL, 14.85 mmol) at 0 0C. After 60 min of stirring, a THF solution of the acetyl chloride (1.31 g, 6.41 mmol) was added at -78 0C. The reaction mixture was stirred at -78 0C for 1 h, then at room temperature for overnight. The resulting reaction solution was quenched with diluted HCl (0.25 M), and the aqueous layer was extracted with Et2O. The combined organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified by flash column chromatography (ethyl acetate/hexanes - 1/9) to give TC-III-85 (0.41 g, 25%) as a yellowish oil.
References:
WO2010/59241,2010,A2 Location in patent:Page/Page column 115